Effect of local anesthetic volume (20 vs. 40 ml) on the analgesic efficacy of costoclavicular block in arthroscopic shoulder surgery: a randomized controlled trial
Article information
Abstract
Background
Among the various diaphragm-sparing alternatives to interscalene block, costoclavicular block (CCB) demonstrated a low hemidiaphragmatic paresis (HDP) occurrence but an inconsistent analgesic effect in arthroscopic shoulder surgery. We hypothesized that a larger volume of local anesthetic for CCB could provide sufficient analgesia by achieving sufficient supraclavicular spreading.
Methods
Sixty patients scheduled for arthroscopic rotator cuff repair were randomly assigned to receive CCB using one of two volumes of local anesthetic (CCB20, 0.75% ropivacaine 20 ml; CCB40, 0.375% ropivacaine 40 ml). The primary outcome was the rate of complete analgesia (0 on the numeric rating scale of pain) at 1 h postoperatively. The secondary outcomes included a sonographic assessment of local anesthetic spread, diaphragmatic function, pulmonary function, postoperative opioid use, and other pain-related experiences within 24 h postoperatively.
Results
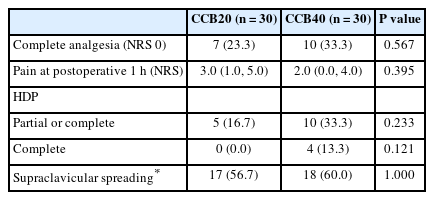
The rates of complete analgesia were not significantly different (23.3% [7/30] and 33.3% [10/30] in the CCB20 and CCB40 groups, respectively; risk difference 10%, 95% CI [–13, 32], P = 0.567). There were no significant differences in other pain-related outcomes. Among the clinical factors considered, the only factor significantly associated with postoperative pain was the sonographic observation of supraclavicular spreading. There were no significant differences in the incidence of HDP and the change in pulmonary function between the two groups.
Conclusions
Using 40 ml of local anesthetic does not guarantee supraclavicular spread during CCB. Moreover, it does not result in a higher rate of complete analgesia compared to using 20 ml of local anesthetic in arthroscopic shoulder surgery.
Introduction
The interscalene brachial plexus block has been the gold standard for shoulder surgery because of its excellent perioperative analgesic efficacy [1]. However, as the course of the phrenic nerve runs close to the brachial plexus at the interscalene level, hemidiaphragmatic paresis (HDP) is almost unavoidable when using this approach [2].
Various diaphragm-sparing alternatives to interscalene blocks have been studied [3]. Among them, costoclavicular block (CCB) that is performed under the clavicle showed reduced HDP with non-inferior analgesic efficacy compared to interscalene block [4]. Recently, a cadaveric study demonstrated that local anesthetic injected into the costoclavicular space can reach the supraclavicular space [5]. Twenty milliliters of dye were injected into the costoclavicular space, spread cephalad to the brachial plexus in the supraclavicular area, consistently reaching the suprascapular nerve and all trunks of the brachial plexus while sparing the phrenic nerve.
However, the promising results of CCB in shoulder surgery do not seem to be reliably reproduced in our clinical experience [6]. In our previous report, the analgesic efficacy of CCB was not consistent, and a few patients required rescue blockades due to severe pain immediately after surgery. As the supraclavicular spreading of local anesthetic and the resulting suprascapular nerve block could be a major determinant for effective analgesia via CCB, we hypothesized that a larger volume of local anesthetic could provide sufficient analgesia by achieving reliable supraclavicular spreading. In this study, we compared the analgesic efficacy of two volumes of local anesthetic (20 vs. 40 ml) for CCB in arthroscopic shoulder surgery.
Materials and Methods
Study design and participants
This single-center, prospective, randomized, parallel-group clinical trial was conducted at the Chungnam National University Hospital, Republic of Korea, and adhered to the tenets of the Declaration of Helsinki, 2013. After obtaining approval from the Chungnam National University Hospital Institutional Review Board (Daejeon, Korea, CNUH IRB 2021-04-068, Chairperson: Prof. Jeong Lan Kim) on June 14, 2021, we prospectively registered the protocol in the Clinical Trial Registry of Korea (KCT0006290, principal investigator: Boohwi Hong) on June 23, 2021 (https://cris.nih.go.kr). Written informed consent was obtained from all participants prior to enrollment.
Patients between the ages of 20 and 80, with American Society of Anesthesiologists (ASA) physical status classifications I–III and scheduled for elective arthroscopic rotator cuff repair between July 2021 and March 2022 (with the first patient enrolled on July 19, 2021) were screened for eligibility. Exclusion criteria encompassed refusal to participate, a body mass index (BMI, kg/m2) of 30 or higher, significant pulmonary disease, sepsis, pregnancy, allergy to amide local anesthetics, infection at the surgical site, history of neck surgery, peripheral neuropathy, chronic pain syndrome, and cognitive impairment. Research data were collected and managed using the Research Electronic Data Capture (REDCap®; https://projectredcap.org) software hosted at Chungnam National University Hospital. REDCap® is a secure web-based platform designed to facilitate data capture in research [7]. This manuscript was written in line with the Consolidated Standards of Reporting Trials guidelines [8].
Randomization and blinding
We utilized a group assigning function in the REDCap® program based on a pre-uploaded sequence of randomization that was generated using blocks of two and four [9]. This was done to conceal the allocation and ensure the allocation sequence was revealed sequentially by a single dedicated researcher (Y.J.) on each case. Patients were randomly assigned to one of the following groups: 20 ml of 0.75% ropivacaine (CCB20) or 40 ml of 0.375% ropivacaine (CCB40). The researcher who conducted the group allocation prepared the study drugs and performed all blocks immediately after the induction of general anesthesia and was excluded from the study thereafter. Other individuals who participated in the surgery and research, including outcome assessors, attending anesthesiologists, surgeons, and nurses, were blinded to the group assignment.
Study flow and anesthetic procedures
Baseline pulmonary function was assessed using a handheld spirometer (CONTECTM SP10 BP Spirometer; Healthcare4all Ltd.) in the ward prior to the day of surgery. On the day of the surgery, patients received premedication with intramuscular midazolam (0.05 mg/kg) before entering the operating room. The diaphragm was assessed using ultrasound as a baseline measurement. General anesthesia was then administered with standard ASA monitoring. Anesthesia induction involved intravenous (i.v.) propofol (1.5 mg/kg), rocuronium (0.8 mg/kg), and remifentanil (1 μg/kg), followed by maintenance with sevoflurane and continuous infusion of remifentanil (0.025–0.2 μg/kg/min) adjusted to maintain blood pressure and pulse rate within ± 20% of baseline. After induction, CCB was performed according to the group allocation. All surgeries were performed by a single experienced surgeon (W.L.) using three portals (posterior, lateral, and minor anterolateral) and one optional anterior portal in the beach chair position. Paracetamol (1 g) and nefopam (20 mg) were administered during surgery. At the end of the surgery, neuromuscular blockade was reversed with 200 mg of sugammadex, and the patients were extubated after confirming adequate ventilation. Immediate postoperative outcomes, including evaluations of the diaphragm and pulmonary functions, upper extremity function, and pain, were assessed sequentially prior to discharge from the post-anesthesia care unit (PACU) with confirmation of a fully cooperative status. Upper extremity assessments were carried out by evaluating hand grip strength (patients were asked to squeeze the investigator’s hand) and hand sensory loss (rubbing the palm and back of the hand). A successful block was defined as the loss of full strength and normal sensation to the light touch in the hand. The overall flow of the study protocol is provided in Supplementary Fig. 1.
Costoclavicular block
Our institution has extensive experience with the CCB, the subject of this study, as demonstrated in our previous studies [10,11]. Patients were placed in a supine position with the ipsilateral limb abducted at an angle of 60°–90°. The infraclavicular area was scanned with an ultrasound probe placed parallel immediately below the clavicle. With gentle tilting to the cephalad direction, the probe was projected toward costoclavicular space, which is defined as the space between the posterior surface of the clavicle and the second rib. Then the image was optimized until the location and relationships of all three cords were identified lateral to the axillary artery [12]. We performed the injections using an in-plane technique and in the lateral-to-medial direction. To ensure the spread of local anesthetic around all three cords of the costoclavicular space, we used separate sequential injections [13,14]. After gently puncturing the paraneural sheath, we dissected the space between the cords using 1–2 ml test doses of injectate while avoiding any swelling signs of the cords. Once we confirmed proper needle placement, we injected 10–15 ml of local anesthetic for the CCB20 group and 20–30 ml for the CCB40 group between the medial and posterior cords. The needle was then slightly withdrawn until its tip was adjacent to the lateral cord, and the remaining volume was injected. All blocks were performed by experienced anesthesiologists using a high-resolution ultrasound system (X-Porte, FUJIFILM SonoSite®, Inc.) with a corresponding high-frequency (15–6 MHz) linear probe (HFL50xp, FUJIFILM SonoSite®, Inc.) and an echogenic needle (SonoPlex®, PAJUNK®). Every injection was divided into small aliquots, each containing 1–2 ml, and any over-pressurization during injection was avoided.
Ultrasound assessment of supraclavicular spreading
First, we performed a pre-procedural scanning on the supraclavicular area for the sequential verifications from the roots, trunk formation, and branching of the suprascapular nerve from the superior trunk [15]. Immediately after the blockade, the supraclavicular area was re-scanned using the same linear probe with minimal contact pressure. Starting from the corner pocket image, where the inferior trunk of the brachial plexus lies on the first rib, the courses of each trunk and suprascapular nerve were traced using cephalad and caudad tilting and the sliding movement of the probe. Adequate spread of the local anesthetic was confirmed by observing the areas where the local anesthetic was visible between the superior and middle trunk or around the suprascapular nerve itself after branching (Supplemental Fig. 2). Other images showing absence or spreading only to the space between the middle and inferior trunks were considered inadequate spreading.
Postoperative pain management
Patient-controlled analgesia (PCA) devices (Accumate®1200, Woo Young Meditech) were used to administer bolus doses of fentanyl 15 μg (10 min of lockout time without basal infusion; total fentanyl dose of 1000 μg). After oral intake was tolerated, all patients received multimodal analgesia consisting of naproxen (500 mg) and tapentadol (50 mg) twice daily. If intolerable pain (numeric rating scale [NRS] ≥ 4) persisted despite these measures, i.v. pethidine (25 mg) was used as rescue analgesia.
Ultrasound assessment of the diaphragm and pulmonary function test
The ultrasound assessment of the diaphragm was performed in a supine position. Two approaches were used for the evaluation of diaphragmatic movement. First, diaphragm excursion (DE) between full inspiration and expiration was measured using M-mode with a 5–2 MHz curved probe (C60xp, FUJIFILM Sonosite®, Inc.). The liver or spleen was used as the acoustic window under the rib at the anterior axillary line. Second, the diaphragm thickness fraction (DTF, %) was assessed at the mid axillary line just inferior to the edge of the pleura with a 15–6 MHz linear probe (HFL50xp, FUJIFILM Sonosite®, Inc.) [16]. DTF was calculated as follows: 100 × (thickness at inspiration - thickness at expiration) / thickness at expiration. Complete HDP was defined by either of the following criteria: 75%–100% decrease in DE, less than 5% in DTF, or occurrence of paradoxical movement of the diaphragm. Partial HDP was defined by either of the following criteria: a 25%–75% decrease in DE or a 5%–20% in DTF. Thus, the absence of any of these criteria is required to indicate the absence of HDP. All ultrasound assessments of the diaphragm were performed by a dedicated researcher (B.H.) blinded to the study groups.
The pulmonary function test was conducted by researchers who were trained in operating handheld spirometers in a semi-recumbent position and were blinded to the group allocation. Forced expiratory volume in 1 s, forced vital capacity, and peak expiratory flow were measured. The third spirometry and ultrasound measurements were recorded after two sets of pre-tests.
Outcome measures
The primary outcome was the rate of complete analgesia (0 on the NRS of pain) at rest 1 h postoperatively. The secondary outcomes included NRS at rest 1 h postoperatively, ultrasound assessment of supraclavicular spreading, time to first use of PCA, postoperative cumulative opioid consumption during 48 h, pain-related experience within 24 h postoperatively assessed with a brief questionnaire, the incidence of HDP, and the changes in pulmonary function.
Data regarding the use of PCA were collected using the AccuLinker (data extraction program of Accumate® 1200 version 1.1; Woo Young Meditech) that records the exact time and dose of every administration performed by the device [17]. The dose of pethidine used as rescue analgesia was converted to 33.3 μg of fentanyl and integrated into the calculation of postoperative cumulative opioid consumption.
Statistical analysis
In a preliminary analysis of our previous study [6], it was observed that approximately 70% of the patients reported a pain score greater than 0 at rest 1 h postoperatively after CCB when 20 ml of local anesthetic was used. We expected that using 40 ml of local anesthetic would demonstrate the incidence of any pain by up to 20%. To detect a difference in the proportion of 50% (70% vs. 20%), with a significance level of 0.05 and a power of 90%, a sample size of 23 patients in each group was calculated. Accounting for a potential dropout rate of about 20%, we planned to include 30 patients in each group.
All statistical analyses were performed using R software, version 4.2.2 (R Foundation for Statistical Computing). Continuous variables were analyzed using the independent t-test (mean ± SD) or Mann–Whitney U test (median [Q1, Q3]), depending on the results of the Shapiro–Wilk tests for the normality of data distribution. Categorical variables were analyzed using χ2 or Fisher’s exact test (expected count < 5) and reported as numbers (%). An alluvial plot was utilized to illustrate the relationship between the volume of local anesthetic (group), supraclavicular spreading of injectate, and postoperative pain. A nonparametric rank-based method was used to analyze the longitudinal change in opioid consumption across different groups [18]. The time to the first dose of PCA was determined using Kaplan–Meier survival analysis and compared using log-rank tests [19]. Factors associated with immediate postoperative pain and HDP were explored using linear and logistic models as appropriate. Statistical significance was set at a two-tailed P value of < 0.05.
Results
From July 19, 2021, to March 29, 2022, 69 consecutive patients were assessed for eligibility. Of these, five were excluded (three for refusal and two for BMI) based on the predetermined criteria. Additionally, four patients were excluded due to changes in the surgical plan (open) before group allocation. The remaining 60 patients were randomly allocated to the CCB20 or CCB40 group. All the enrolled patients were included in the final analysis (Fig. 1). The baseline patient and clinical characteristics are shown in Table 1. There were no failed blocks according to the predefined criteria. No serious block-related complaints or complications such as dyspnea, desaturation, and pneumothorax were observed during the trial.
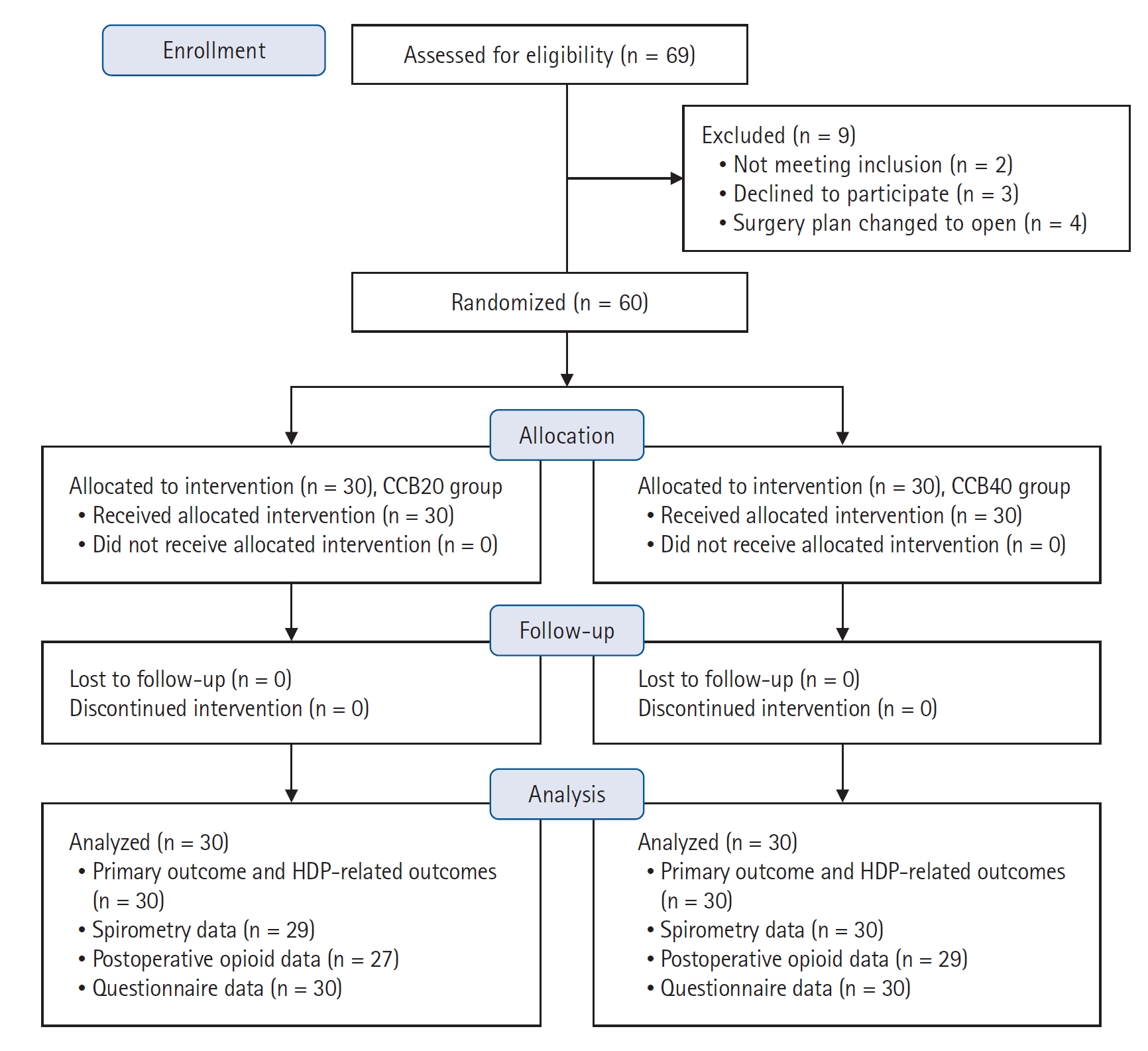
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. CCB20 group: costoclavicular block with 20 ml of 0.75% ropivacaine, CCB40 group: CCB with 40 ml of 0.375% ropivacaine. HDP: hemidiaphragmatic paresis.
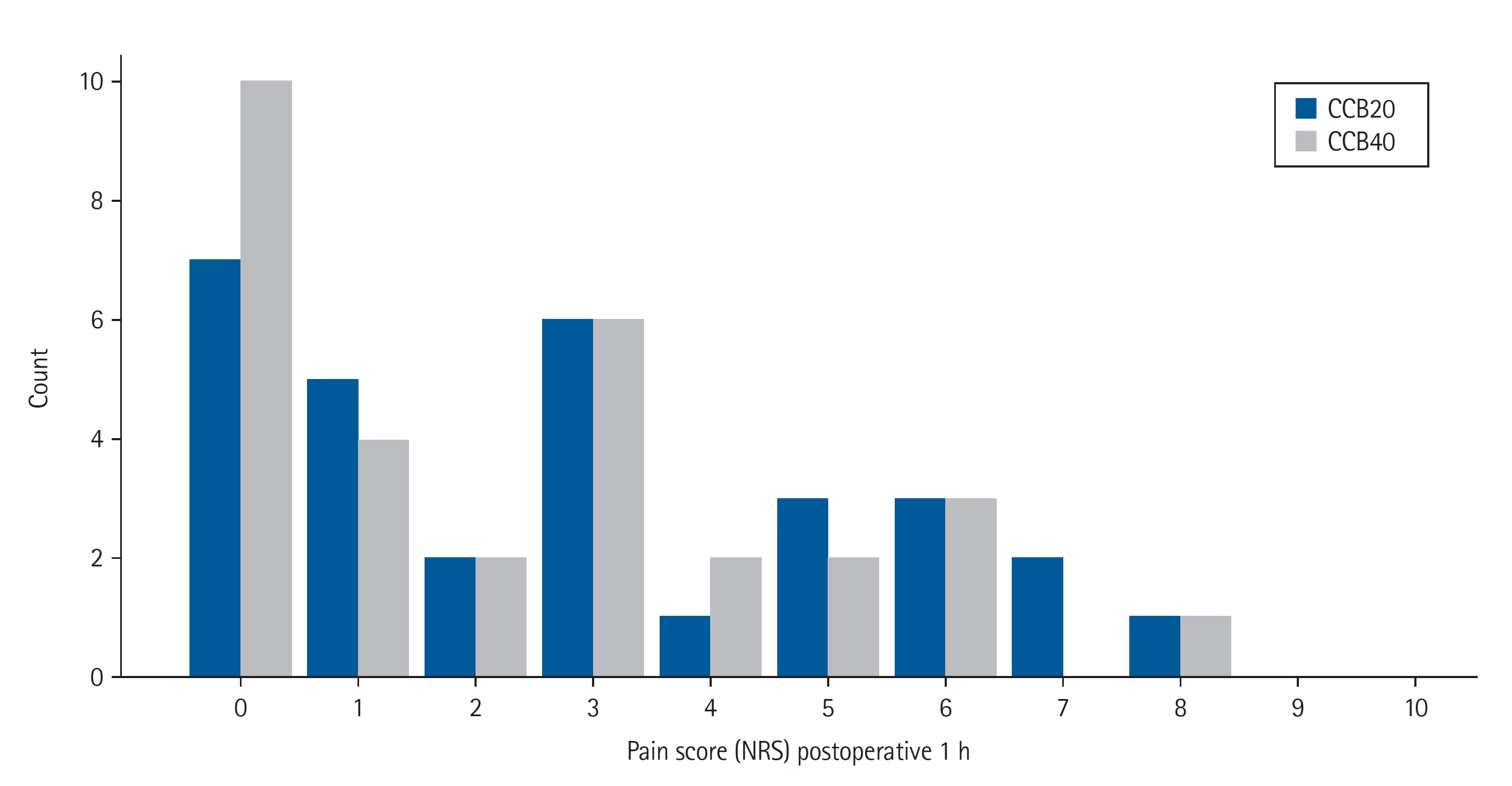
The rate of complete analgesia, indicated by 0 on the NRS, at the PACU was 23.3% (7/30) in the CCB20 group and 33.3% (10/30) in the CCB40 group (risk difference 10%, 95% CI [–13, 32], P = 0.567; Fig. 2). The pain score at 1 h was not significantly different between the groups (3 [1, 5] in CCB20 vs. 2 [0, 4] in CCB40, P = 0.395; Table 2). Sonographic assessment of the supraclavicular spreading showed no significant difference between the groups. The association between the groups, supraclavicular spreading, and immediate postoperative pain are shown in Fig. 3. While most cases with complete analgesia were associated with adequate spreading of local anesthetic, complete analgesia was not always achieved despite adequate spreading. Univariable and multivariable linear regression analyses showed that ultrasound observation of supraclavicular spreading was the only significant factor associated with immediate postoperative pain score (Table 3).
Primary Outcome (Complete Analgesia), Incidence of HDP, and the Evaluation of Supraclavicular Spreading of Local Anesthetic

Alluvial plot for relation of group, supraclavicular spreading of local anesthetic, and complete analgesia in immediate postoperative period. Note that while most cases with complete analgesia were associated with the adequate supraclavicular spreading of local anesthetics, complete analgesia was not always achieved despite adequate supraclavicular spreading. CCB20: costoclavicular block with 20 ml of 0.75% ropivacaine, CCB40: CCB with 40 ml of 0.375% ropivacaine.
There were no significant differences in the results of the pain-related questionnaire between the groups (Supplementary Table 1). No significant difference was observed in the cumulative opioid consumption between the groups during the postoperative period (P = 0.627; Supplementary Fig. 3). In addition, no significant interaction was found between the groups and the measurement time points (P = 0.371; Supplementary Fig. 3).
Time to first request of analgesia did not differ significantly (median 6.9 h, 95% CI [2.9, 16.1] h in CCB20 vs. 5.5 h, 95% CI [3.7, 13.4] h in CCB40, P = 0.640; Supplementary Fig. 4).
DE data from one patient in the CCB40 group (left-sided procedure) was excluded because of difficulty in visualization. DTF was evaluated in all patients. Complete HDP was observed in four patients in the CCB40 group and zero in the CCB20 group that was not significantly different (P = 0.121; Supplementary Table 2). The univariable analysis did not reveal any significant predictors for the occurrence of HDP (partial or complete; Supplementary Table 3).
The pulmonary function test of one patient in the CCB20 group was withheld due to poor cooperation during the examination. No significant differences between the groups were observed in the reduction rates of pulmonary function (Supplementary Table 4).
Discussion
In this trial, we hypothesized that the larger the volume of local anesthetic used during CCB, the greater the spread into the supraclavicular area, thereby achieving complete analgesia during arthroscopic shoulder surgery. However, we found that neither adequate spreading of the local anesthetic nor complete analgesia was guaranteed by a larger volume of local anesthetic. In addition, despite its significant association with lower pain scores, the sonographic finding of supraclavicular spreading of local anesthetic was not a reliable indicator of complete analgesia.
The density of nociceptors in the shoulder joint is highest in the subacromial bursa, anterior glenohumeral capsule, and ligaments that are innervated by the suprascapular, axillary, lateral pectoral, and upper subscapular nerves [20,21]. The lateral pectoral (lateral cord), subscapular (posterior cord), and axillary (posterior cord) nerves can be directly covered by a CCB. Meanwhile, the suprascapular nerve branching off from the superior trunk can only be blocked by the supraclavicular spreading of the local anesthetic [5]. Thus, the severe pain that manifested in several patients in this study can be partially explained by inadequate supraclavicular spreading. Additionally, the lateral pectoral nerve can also be spared by inadequate spreading. According to a previous meta-analysis, the lateral pectoral nerve arises most frequently from the anterior divisions of the upper and middle trunks before forming the cords [22].
However, it should be noted that while supraclavicular spreading determined by post-procedure ultrasound showed a certain degree of association with lower pain score, it did not guarantee complete analgesia in this study. There could be several explanations for this. Firstly, the binary determinations of adequate versus inadequate spreading in sonographic imaging and complete versus incomplete analgesia may not be realistic classifications. This stringent classification may have limitations in representing a more nuanced spectrum of local anesthetic spreading and the corresponding quality of blockade. Secondly, in some cases, the suprascapular nerve can branch off early and course further away from the plexus. This might explain why some patients still suffer from pain despite being determined to have proper local anesthetic spread between the superior and middle trunk. Thirdly, there may be at least some degree of ambiguity and/or subjectivity in the visual evaluation of sonographic findings. Unfortunately, the exact cause of insufficient shoulder analgesia cannot be concluded in this study. Further research using detailed assessments of each major contributing nerve after CCB is needed [23].
According to previous dose-finding studies of CCB, approximately 20 ml of local anesthetic is required for surgical anesthesia in forearm and hand surgeries [24,25]. Since even twice the volume of injectate during CCB showed no significant improvement in supraclavicular spreading in the current study, other factors affecting proper coverage of the shoulder joint should be considered. One of the possible factors is the intraplexus fascial septum [14] that separates the two compartments (i.e., either the anterior that contains the lateral cord, or the posterior that contains the medial and posterior cord) and may affect spreading. Because we injected a larger volume into the posterior compartment, whether supraclavicular spreading is better if a larger volume is injected into the anterior compartment needs to be confirmed. In addition, as shown in this study, direct observation of the supraclavicular spread of the injectate via ultrasound would be helpful, and additional measures to supplement inadequate coverage (e.g., suprascapular nerve block) can be sought in advance.
Although the costoclavicular space is contiguous with the supraclavicular space, CCB has shown a lower HDP rate than the supraclavicular block in previous studies [10,11,26]. However, the occurrence of HDP (4%–11% incidence) is not completely avoidable with CCB. In line with the insignificant volume effect on supraclavicular spreading, the HDP rate was irrelevant to the injectate volume. Although complete HDP was observed only in CCB40, no statistical significance in group difference was found. Also, supraclavicular spreading was not a significant determinant of HDP either. However, since HDP is not the main outcome of this study, it cannot be confirmed, and additional well-designed studies are needed.
While it is true that CCB is a diaphragm-sparing technique when compared to other approaches performed above the clavicle [27], caution should be exercised when comparing studies due to the differences in the definition of HDP used in each study. Our findings appear to conflict with a prior study that reported no cases of HDP when utilizing CCB in shoulder surgery [4]. However, the definition of HDP in that study only focused on a very severe case of complete HDP, absence, or paradoxical movement of the diaphragm that makes it challenging to compare with the results of other studies that define complete HDP as less than 25% of the baseline DE value.
Anatomical variations in the phrenic nerve can contribute to varying degrees of partial HDP. Duplication or lateral displacement of the phrenic nerve and its accessory variations may result in variable degrees of HDP due to supraclavicular spreading during CCB [28,29]. Therefore, it may be important to distinguish whether the main branch of the phrenic nerve that originates from C4 is blocked. From this perspective, using all-or-nothing criteria of prior study may be more clinically relevant [4].
This study has several limitations that should be considered when interpreting the results. First, the 40 ml volume of local anesthetic may not be large enough for supraclavicular spreading. Second, the primary outcome of the zero-pain score may have been too strict. Considering the benefit of the low risk of HDP after CCB, mild pain that is well tolerated by the patient may be acceptable, especially in patients with limited respiratory capacity. Third, intermediate or superficial cervical plexus block was not performed in this study. During interscalene or supraclavicular blocks, the C4 dermatome can also be blocked by superior spreading, which is hardly expected during CCB [30]. Although the cape region that is covered by the cervical plexus is not the main source of pain in arthroscopic shoulder surgery, the incomplete analgesia reported in this study may be partially explained by this issue. Fourth, the results cannot be generalized to other shoulder procedures, such as total shoulder replacement or fixation of humerus fractures that might follow different healing processes and consequent pain trajectories. Fifth, bias due to the disclosure of group assignment to the physician who performed the blockades cannot be excluded. Lastly, given that the evaluation of lung function took place after general anesthesia, differentiating the distinct impact of the block from that of general anesthesia might present a challenge.
In conclusion, 40 ml of local anesthetic does not guarantee supraclavicular spread during CCB and does not show a greater rate of complete analgesia than 20 ml of local anesthetic in arthroscopic shoulder surgery. Due to the inconsistent analgesic effects observed even with a high volume of local anesthetic, CCB does not seem to be an ideal analgesic technique for shoulder surgery. Further research exploring techniques that provide both effective analgesia and diaphragm-sparing in shoulder surgery is needed.
Notes
Funding
This work was supported by the National Research Foundation of Korea (NRF-2022R1C1C1007982) of Chungnam National University.
Conflicts of Interest
Woosuk Chung has been an editor for the Korean Journal of Anesthesiology since 2020. However, he was not involved in any process of review for this article, including peer reviewer selection, evaluation, or decision-making. There were no other potential conflicts of interest relevant to this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Yumin Jo (Conceptualization; Data curation; Project administration; Writing – original draft)
Chahyun Oh (Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing)
Woo-Yong Lee (Methodology; Project administration; Resources)
Hyung-Jin Chung (Investigation; Methodology; Project administration)
Hanmi Park (Data curation; Project administration)
Juyeon Park (Data curation; Project administration)
Jieun Lee (Data curation; Project administration; Resources)
Yoon-Hee Kim (Supervision; Writing – review & editing)
Youngkwon Ko (Supervision; Writing – review & editing)
Woosuk Chung (Resources; Supervision; Validation; Writing – review & editing)
Boohwi Hong (Conceptualization; Formal analysis; Funding acquisition; Software; Visualization; Writing – original draft; Writing – review & editing)
Supplementary Materials
Study protocol. Diaphragm evaluation using ultrasound and spirometry was performed before and after surgery.
Ultrasound-guided observation of brachial plexus at the supraclavicular area. (A) Pre-block, the suprascapular nerve lies laterally within the superior trunk at the corner pocket image. (B) Local anesthetic is spread around trunks and suprascapular nerve after the costoclavicular block.
Cumulative opioid consumption during the postoperative 48 hours.
The proportion of patients not requiring the first dose of patient-controlled analgesia (PCA) stratified by group.
Questionnaire about the experience at 24 hours postoperatively stratified by group.
Sonographic assessments of diaphragm function.
Logistic regression analysis for factors associated with hemidiaphragmatic paresis (HDP).
Spirometry results stratified by group.