 |
 |
| Korean J Anesthesiol > Volume 77(3); 2024 > Article |
|
Abstract
Background
Methods
Results
NOTES
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Jongchan Lee (Data curation; Formal analysis; Investigation; Visualization; Writing – original draft)
Sujung Park (Formal analysis; Investigation; Validation; Writing – review & editing)
Jae Geun Lee (Data curation)
Sungji Choo (Investigation; Visualization; Writing – original draft)
Bon-Nyeo Koo (Conceptualization; Project administration; Supervision; Writing – review & editing)
Fig. 1.
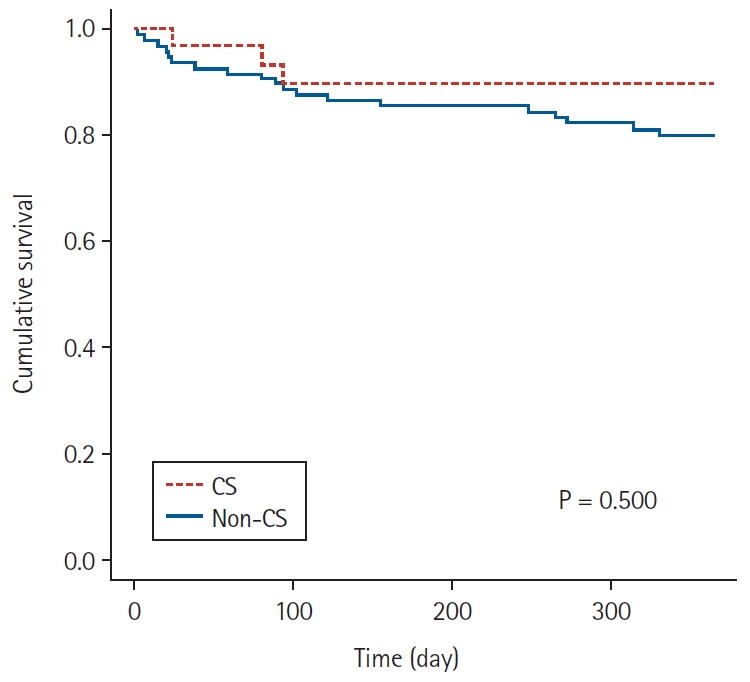
Table 1.
Values are presented as numbers (%), mean ± SD or median (Q1, Q3), except for categorical variables, for which values represent numbers of patients with percentages. CS: Cell Saver, SMD: standardized mean difference, BMI: body mass index, MELD: model for end-stage liver disease, ASA: American Society of Anesthesiologists classification, ESLD: end-stage liver disease, AST: aspartate transaminase, ALT: alanine transaminase, PT: prothrombin time, INR: international normalized ratio, aPTT: activated partial thromboplastin time.
Table 2.
Table 3.
Values are presented as median (Q1, Q3) or mean ± SD. CS: Cell Saver, POD: postoperative day, INR: international normalized ratio, aPTT: activated partial thromboplastin time, RBC: red blood cell, FFP: fresh frozen plasma, ICU: intensive care unit. *Postoperative transfusion: the total amount of transfused blood from admission to the surgical intensive care center until discharge.









