|
 |
| Korean J Anesthesiol > Volume 77(2); 2024 > Article |
|
Abstract
Background
Methods
Results
Acknowledgments
NOTES
Funding
This study was supported by an institutional research grant from Seoul National University Hospital (Grant No. 0420222160).
Data Availability
The data that support the findings of this study are available from the Korea National Health Insurance Service but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the corresponding author upon reasonable request and with permission of the Korea National Health Insurance Service.
Author Contributions
Jae-Woo Ju (Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft)
Taeyup Kim (Data curation; Formal analysis)
Soo-Hyuk Yoon (Data curation; Formal analysis)
Won Ho Kim (Formal analysis; Writing – review & editing)
Ho-Jin Lee (Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Supervision; Writing – review & editing)
Supplementary Materials
Supplementary Table 1.
Supplementary Table 4.
Supplementary Table 5.
Supplementary Table 6.
Supplementary Table 7.
Supplementary Table 8.
Supplementary Table 9.
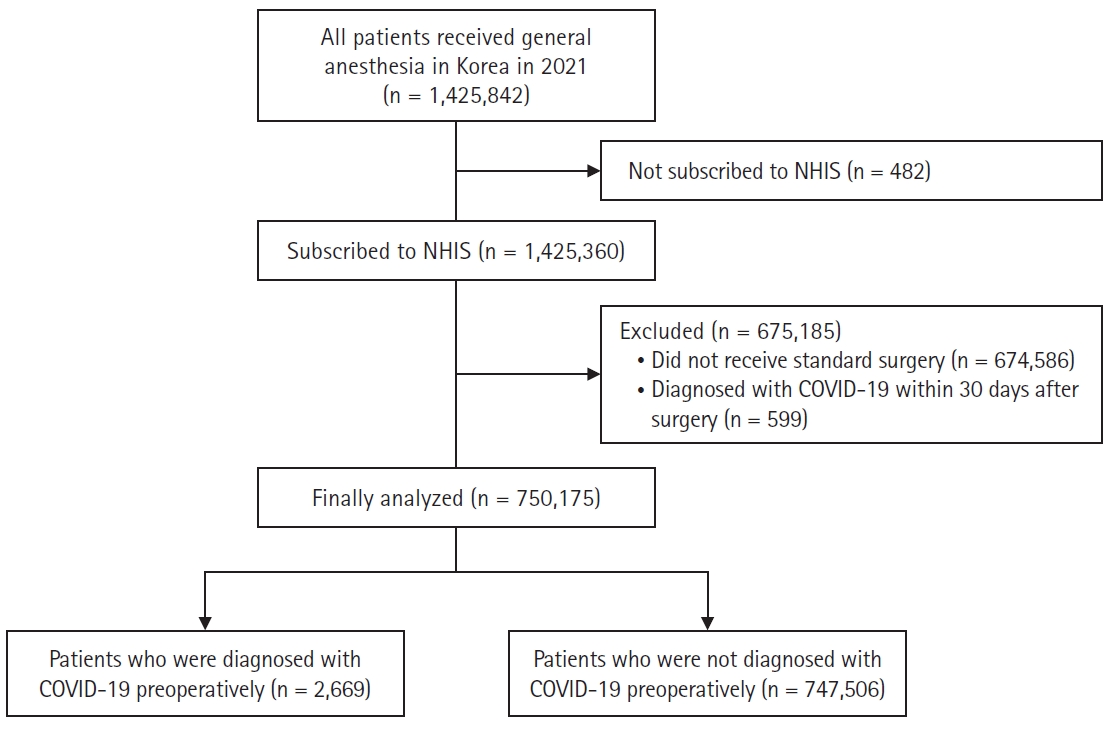
Fig. 1.
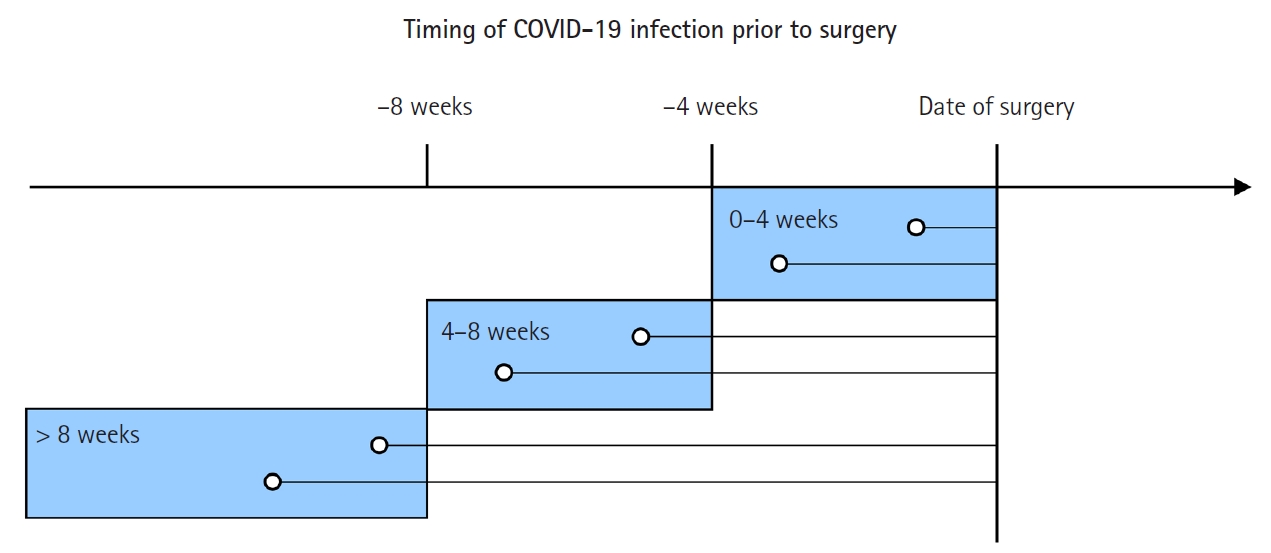
Fig. 2.