|
 |
|
|
Abstract
A 56-year-old man on maintenance hemodialysis was admitted to the intensive care unit with septic shock and coagulopathy. As there was a dialysis catheter in the right internal jugular vein, the left internal jugular vein was cannulated with a central venous catheter to initiate vasopressor therapy. A chest X-ray showed formation of a catheter loop inside the left brachiocephalic vein, probably due to hindrance by the dialysis catheter. This report describes the hurdles encountered, repeated cannulation attempts, and serial chest X-ray findings required to obtain acceptable placement of the catheter tip.
A 56-year-old man with chronic kidney disease on maintenance hemodialysis developed septic shock with coagulopathy. As there was a dialysis catheter in the right internal jugular vein, we attempted to cannulate the left internal jugular vein with a standard triple-lumen central venous catheter. However, this repeatedly ended up malpositioned, probably after hitting the dialysis catheter on its way. This paper discusses the practical concerns encountered with the repeated failed attempts and serial chest images in a bid to place the catheter tip in an acceptable position. The patient consented to publication of this case study.
A 56-year-old man was transferred from the dialysis unit to our intensive care unit (ICU) with altered sensorium and hypotension. He had chronic kidney disease and had been on maintenance hemodialysis for several months.
On ICU admission, his Glasgow coma scale (GCS) score was 7/15: E2 (eye response), V2 (verbal response), and M3 (motor response). The heart rate was 112 beats/min, non-invasive blood pressure was 87/48 mmHg, and axillary temperature was 38.5℃. A probable diagnosis of dialysis-related septic shock was made and fluid resuscitation, routine cultures, and empirical antibiotic therapy were initiated. Considering the poor sensorium and unstable hemodynamics, he was intubated electively and put on mechanical ventilation.
His laboratory parameters were unremarkable, except a leukocyte count of 17 × 106/cm3, sodium levels of 122 mEq/L in serum, potassium of 3.2 mEq/L, and international normalized ratio of 3.2 with a normal platelet count.
To initiate vasopressor therapy to support the hemodynamics, we considered using the dialysis catheter already placed in the right internal jugular vein (IJV). After discussing this with the nephrology team, it was decided to put a separate central venous catheter in the left IJV to administer vasopressors without hindering dialysis and to monitor the central venous pressure (CVP). In addition, the dialysis catheter was considered the source of the sepsis.
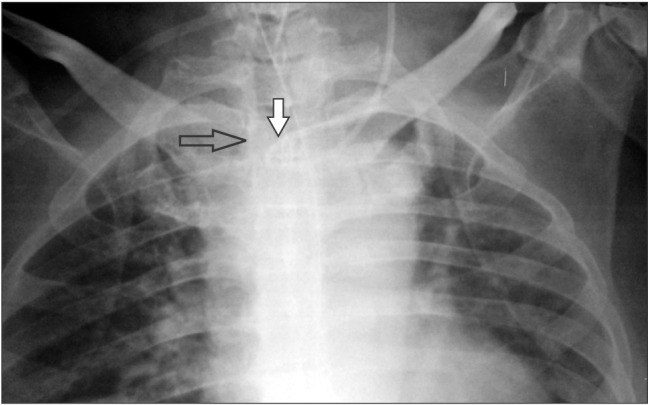
Ultrasound-guided insertion of a catheter (B-Braun Certofix Trio V720, 7F) into the left IJV was successful up to the 12 cm mark on the skin surface. The post-procedure chest X-ray revealed that the catheter had formed a loop inside the left brachiocephalic vein with its tip in the left subclavian vein (Fig. 1).
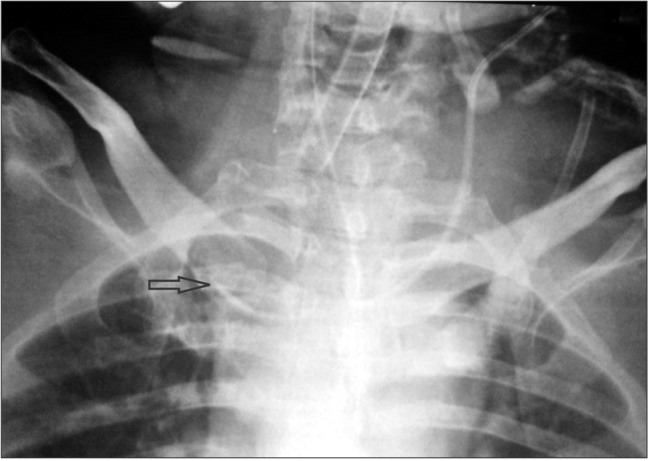
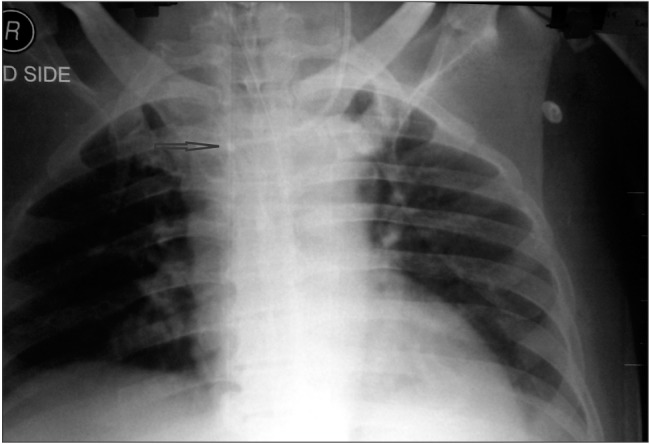
The dialysis catheter in the right IJV was considered a possible hurdle to the proper placement of the triple-lumen catheter. Because the dialysis catheter was also considered a possible source of infection, it was removed and a new one was put in a femoral vein. After removing the dialysis catheter, the central line was repositioned using the guidewire. A follow-up chest X-ray showed that the catheter had entered the right subclavian vein (Fig. 2). After observing the X-ray, we decided to withdraw the catheter by 1–2 cm so as to place it at the junction of the great veins (Fig. 3). This was to reduce additional manipulations and come to an acceptable compromise regarding the position of the catheter tip.
A patient was admitted to our ICU with septic shock, and we felt an urgent need for central venous access to infuse vasopressors and monitor CVP. We considered using the double-lumen dialysis catheter already inserted into the right IJV, but the patient was dependent on the dialysis and required it frequently. Furthermore, we could not rule out the possibility that the dialysis catheter itself was the source of infection. After discussion with nephrologists, we decided to insert a separate, exclusive central venous catheter.
The alternative sites available were right or left subclavian, left internal jugular, or either femoral vein. As the patient's coagulation parameters were abnormal, a subclavian approach was avoided because the subclavian veins are not amenable to external pressure when they bleed, unlike IJVs [1]. Although both femoral sites were available, the reliability of femoral vein CVP readings as a surrogate is controversial, except when the intra-abdominal pressure is < 15 mmHg [2]. Pacheco et al. [3] found a linear relationship between the superior vena cava and femoral vein readings when the head is positioned at zero degrees immediately after cardiac surgery. In contrast, a similar study by Groombridge et al. [4] found poor agreement between the femoral and central venous pressures. In terms of complications, femoral vein cannulation averts some dreaded complications such as injury to the pneumothorax and carotid artery, whereas infectious complications and venous thromboembolism are more frequently associated with it being closer to the groin [5,6,7]. Therefore, we did not choose a femoral route and the left IJV approach remained.
Next, we considered whether one can cannulate both IJVs in a patient at the same time. The literature does not favor bilateral IJV cannulation at the same time with large-bore cannulas, particularly in patients with elevated intracranial pressure (ICP) [8]. Although our patient had no evidence of an elevated ICP, he was in shock with possibly decreased cerebral perfusion. After weighing the associated possible risk of compromising cerebral venous return and the benefit of infusing vasopressors instantly, we opted for left IJV cannulation with a triple-lumen catheter.
Misplacement or malposition of a central venous catheter is not an unusual phenomenon in clinical practice. It usually occurs during attempts that are made from the left side because of the acute angulation and comparatively difficult anatomy, with a higher chance of injuring the left lung cupola and thoracic duct. Misplacement can be intravascular or occasionally extravascular, which can result in worse complications. To add to the complexity, misplacement can occur at either the time of catheter insertion or any later time. The ideal catheter tip position is also controversial. The literature favors having the tip at least in a large central vein and as close to the right atrium as possible. The tip has to be proximal to the pericardial sac and venous valves to enable a correct CVP measurement without causing tamponade.
During the initial insertion, the catheter probably hit the dialysis catheter and formed a loop inside the left brachiocephalic vein. Subsequently, when it was manipulated with the help of a guidewire and the dialysis catheter was removed, the catheter went straight into the right subclavian vein. To avoid repeated manipulations, which might enhance the risk of infection, we withdrew the catheter slightly and left it in the superior vena cava, as an acceptable compromise.
The complication rate is lower when a catheter is inserted with ultrasound guidance [9,10]. Nevertheless, catheter tip malposition is not completely avoidable at either the time of insertion or a later date, even with the use of ultrasound.
In our situation, the best practice might have been to remove the dialysis catheter first, and then attempt to cannulate the IJV. However, we could not do this because the patient would have required insertion of another dialysis catheter at an alternative site on the same day and we only suspected, but had not yet proven, that there was a dialysis catheter-related bloodstream infection. Furthermore, it was possible that our attempts to cannulate a central vein might fail and in these circumstances the in situ dialysis catheter could have been used as a lifesaving central conduit in an emergency.
We were unable to determine the exact cause of the catheter misplacement. One might consider using fluoroscopy or venography to confirm or exclude any superior vena cava stenosis or to prove that the dialysis catheter was the true cause of the mishap. In addition, a contrast agent could have been instilled under fluoroscopic guidance and catheter coiling or an iatrogenic venous thrombosis could well have been observed, as stated by Danckers et al. [11]. However, our patient was in the ICU, had no signs of superior vena cava syndrome, and was in shock and renal failure, which created restrictions.
In conclusion, we suggest that expedited radiographic confirmation of the position of the catheter and its tip be obtained if such a situation arises in clinical practice, and we emphasize the need for serial X-rays.
References
1. Hocking G. Central venous access and monitoring. Update Anaesth 2000; 12: 1-6.
2. Caramelo N, Gonçalves P, Paisana A, Silva B, Dias C, Severino S, et al. Central venous pressure in a femoral access: a true evaluation? Crit Care 2007; 11(Suppl 2): 277.

3. Pacheco Sda S, Machado MN, Amorim RC, Rol Jda L, Corrêa LC, Takakura IT, et al. Central venous pressure in femoral catheter: correlation with superior approach after heart surgery. Rev Bras Cir Cardiovasc 2008; 23: 488-493. PMID: 19229419.


4. Groombridge CJ, Duplooy D, Adams BD, Paul E, Butt W. Comparison of central venous pressure and venous oxygen saturation from venous catheters placed in the superior vena cava or via a femoral vein: the numbers are not interchangeable. Crit Care Resusc 2011; 13: 151-155. PMID: 21880001.


6. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med 2007; 35: 1390-1396. PMID: 17414086.


7. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003; 348: 1123-1133. PMID: 12646670.


8. Stocchetti N, Longhi L, Valeriani V. Bilateral cannulation of internal jugular veins may worsen intracranial hypertension. Anesthesiology 2003; 99: 1017-1018. PMID: 14508338.


9. Randolph AG, Cook DJ, Gonzales CA, Pribble CG. Ultrasound guidance for placement of central venous catheters: a meta-analysis of the literature. Crit Care Med 1996; 24: 2053-2058. PMID: 8968276.


10. Karakitsos D, Labropoulos N, De Groot E, Patrianakos AP, Kouraklis G, Poularas J, et al. Real-time ultrasound-guided catheterisation of the internal jugular vein: a prospective comparison with the landmark technique in critical care patients. Crit Care 2006; 10: R162PMID: 17112371.



Fig. 1
A chest X-ray showing the right internal jugular vein dialysis catheter in situ and the loop formed by the central venous catheter inserted into the left internal jugular vein.