|
 |
|
|
Abstract
Background
The primary outcome of sugammadex reversal for rocuronium-induced neuromuscular block (NMB) is a train-of-four ratio (TOFR) of 0.9, not first twitch (T1) height. We investigated whether the recovery of TOFR or T1 differs based on the reversal of NMB with neostigmine or sugammadex.
Methods
The acceleromyographic responses from 0.6 mg/kg of rocuronium were monitored supramaximally in 80 patients after induction of anesthesia. The TOFR and T1 height were recorded, and saved in a personal computer using TOF-Watch SX Monitor software in all patients. Patients were randomly assigned to 2 groups to receive either neostigmine 50 µg/kg with glycopyrrolate 10 µg/kg (neostigmine group, n = 40) or sugammadex 2.0 mg/kg (sugammadex group, n = 40). The primary objective was to determine the difference of recovery time between TOFR to 0.9 and T1 to 0.9 after sugammadex or neostigmine administration during moderate rocuronium-induced NMB.
Exact monitoring of muscle relaxation is essential for proper use of muscle relaxants and to prevent post-operative residual paralysis [1]. Train-of-four (TOF) monitoring and single twitch (ST) stimulation of peripheral nerves is often used to monitor the degree of neuromuscular block (NMB) when anesthetic techniques include use of neuromuscular blocking agents (NMBAs). Using ST should establish a control value before the administration of NMBAs. ST and first twitch of TOF (T1) forces do not differ when employing a stimulus interval of 10 seconds (s) or more [2]. The T1 response represents the effects of NMBAs in the postsynaptic membrane, while the TOF ratio (T4/T1, TOFR) shows the effect on the pre-synaptic membrane of the neuromuscular junction [3].
During spontaneous or pharmacologically-induced offset of NMB, a TOFR of 0.9 has been reported to exclude clinically important residual NMB [3]. The T1 usually recovers at a rate similar to or faster than the TOFR after the reversal of competitive NMBAs by neostigmine. After reversal with the optimum dose of sugammadex during rocuronium-induced NMB, the time to 0.9 of the TOFR is much faster than that to 90% of T1 (T1 / control value). A TOFR of 0.9 cannot assure adequate recovery of neuromuscular function in several minutes after sugammadex administration, if the T1 was not recovered completely [4,5]. However, this previous report simply described the time of a T1 and TOFR to 0.9, not saved in a personal computer using TOF-Watch SX Monitor software. So the detailed relationship between a TOFR and T1 should be analyzed following the time after sugammadex administration.
We analyzed the relationship of T1 recovery and TOFR after administration of sugammadex 2.0 mg/kg or neostigmine 50 µg/kg during rocuronium-induced moderate NMB.
After approval of the protocol by the Hospital Ethics Committee, 80 adult patients were enrolled in this study and all granted written informed consent. This randomized, parallel-group, blinded study was conducted in accordance with principles of Good Clinical Research Practice [3]. All patients of both sexes, American Society of Anesthesiologists physical status I–II, aged between 20–64 years, received elective surgery under general anesthesia with rocuronium for intubation and maintenance. Using a computer-generated program, patients were randomly assigned to either the neostigmine group (n = 40, neostigmine 50 µg/kg with glycopyrrolate 10 µg/kg after operation) or the sugammadex group (n = 40, sugammadex 2.0 mg/kg after operation) (Fig. 1). Exclusion criteria were as follows: predicted difficult intubation, previous known neuromuscular diseases that may affect NMB, allergy to any drug used in general anesthesia, history of serious liver or kidney disease, use of drugs that might interact with NMBAs, pregnancy, or obesity (defined as a body mass index [BMI] ≥ 30 kg/m2).
In the operating room, the patients received a standard monitoring of pulse oximetry, capnography, electrocardiography and noninvasive blood pressure measurement. Depth of hypnosis was evaluated for all patients using a bispectral index (BIS) XP monitor (Model A 2000, Aspect Medical Systems, Newton, MA, USA). Anesthesia was induced without premedication, using 2.0–2.5 mg/kg propofol, 0.5 µg/kg/min remifentanil, and oxygen, and was maintained with 1.0–1.5 MAC of sevoflurane in 50% air in oxygen and 0.1–0.3 µg/kg/min remifentanil to maintain a BIS monitor reading between 40 and 50 throughout surgery. During maintenance of anesthesia, end-tidal PCO2 was kept between 30 and 35 mmHg.
NMB was monitored using a TOF-Watch SX® (Merck Sharp & Dohme Corp., Glostrup, Denmark) at the adductor pollicis muscle. Two surface electrodes (Cleartrode™, ConMed®, Utica, NY, USA) were placed on cleaned skin overlying the ulnar nerve at the wrist. Stabilization was achieved with a 5 s, 50 Hz tetanic stimulation. Supramaximal stimuli were applied after automatic calibration (CAL 2 mode) of acceleromyography after an initial tetanic stimulus [3]. If first TFR and T1 were < 95% or > 105%, we performed the recalibration after the change of the location of a pizoelectric transducer or fixed wrist. This experiment was begun after confirming a TFR and T1 of 95%–105%. The TOF mode of supramaximal stimulation (0.2 ms duration, frequency 2 Hz, 2 s duration) was applied at 15 s intervals, which lasted until the end of anesthesia. After stabilization of control responses, rocuronium was administered at 0.6 mg/kg before tracheal intubation, then subsequently at 0.2 mg/kg when the second twitch (T2) reappeared in TOF stimulation.
The primary objective of this study was to determine recovery time and response after sugammadex or neostigmine administration of the T1 and TOFR to 0.9 during rocuronium-induced moderate NMB. After the surgical dressing, each patient received sugammadex 2.0 mg/kg or a single dose of neostigmine 50 µg/kg plus glycopyrrolate 10 µg/kg, respectively, when T2 reappeared in TOF stimulation. All neuromuscular monitoring data were saved in a personal computer using TOF-Watch SX Monitor software. Full recovery from NMB was conducted during the administration of sevoflurane and remifentanil. Extubation was carried out after consciousness and regular respiration were confirmed. Skin temperature of the hand was measured and kept above 32℃. Central temperature was continuously monitored at the lower esophagus and kept above 35.5℃.
Statistical analysis was performed with SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Sample size was determined based on the difference of recovery time (mean [SD] recovery times of 4.4 [7,9] min) between TOFR to 0.9 and T1 to 0.9 after sugammadex 4 mg/kg during deep rocuronium-induced NMB [5]. We considered time reduction by more than 40%, to 2.6 min (SD 3.2), to be clinically significant. Obtaining statistically significant results with α = 0.05 and a power of 0.8 required 37 patients. Projecting 10% dropout, we enrolled 40 patients in each group. Characteristics of patients were compared using the Chi square test between the two groups, and T1 height and TOFR were compared using an unpaired t-test. Repeated measures analyses of variance (ANOVA) were conducted for identifying the neuromuscular recovery. Data are expressed as the mean ± SD, range, or number. Differences were considered to be significant at P < 0.05.
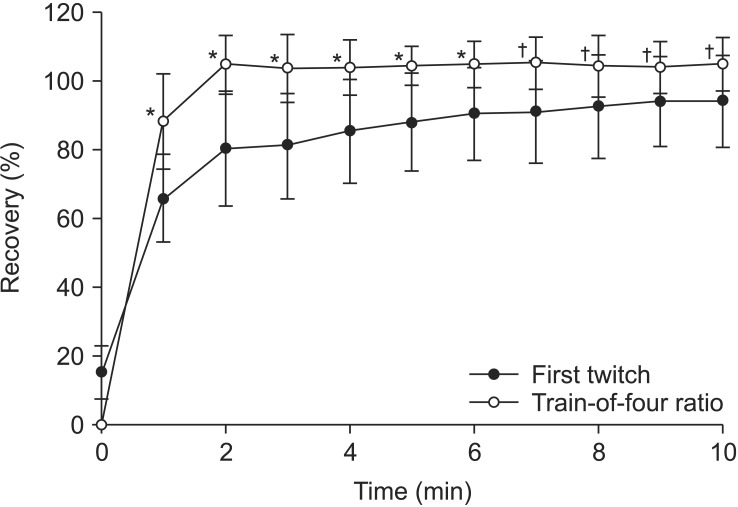
All enrolled patients completed the study, and neuromuscular monitoring was successfully conducted. The two groups were well balanced in their demographic profile, end-tidal concentration of sevoflurane and anesthetic time (Table 1). The T1 recovery and TOFR was 87.8% ± 17.5% and 90.1% ± 12.1% at 15 min after neostigmine 50 µg/kg, respectively (P = 0.496). The recovery pattern of T1 and TOFR was similar during the 20 min after neostigmine administration (Fig. 2). The T1 recovery and TOFR was 90.4% ± 13.5% and 104.9% ± 6.7% at 6 min after sugammadex 2 mg/kg, respectively (P < 0.001). The recovery pattern of TOFR after 2 min was 100% or more, but that of T1 was less than 90% up to 6 min after sugammadex administration (Fig. 3). Of particular note, the neuromuscular recovery of T1 at 120 s following administration of sugammadex was only 80.2% ± 16.7%, but the TOFR at the same time was 104.8% ± 8.4% (P < 0.001) (Table 2). The T1 recovery was 12.3% ± 6.1% and 15.2% ± 7.8% in the neostigmine and sugammadex groups, respectively, at time 0 when the second twitch was observed in TOF stimulation (P = 0.257) (Figs. 2 and 3).
We found that the recovery pattern of the TOFR after 2 min was 1.0 or more, but that of T1 was less than 90% up to 6 min after sugammadex administration. In particular, the neuromuscular recovery of T1 was only 80% compared with a TOFR of 1.0 at 120 s. The recovery pattern of T1 and TOFR was similar during the 20 min after neostigmine administration.
Postoperative residual paralysis is frequent, dangerous, and difficult to recognize clinically. An impaired ventilatory response to hypoxemia, visual impairment and the possibility of increased risk of aspiration occurs when the TOFR is < 0.9. Patients are likely to feel an unpleasant feeling of residual paralysis in a short time [6]. Even with recovery to a TOFR of more than 0.9, the incidence of pharyngeal dysfunction is 13% [7]. It is recommended that a TOFR of more than 0.9 should be confirmed before extubation using acceleromyographic monitoring.
The relationship between the absolute value of T1 height and the TOFR is constant and reliable as a method of monitoring the effects of NMBA and their antagonists [8]. When T1 height has returned to a control value, the recovery pattern of TOF stimulation frequently fades markedly (indicating residual receptor occupancy). T1 recovery to 90% is similar to a TOFR of 0.7, representing blockage of 80% of neuromuscular receptors after anticholinesterase or spontaneous recovery [9,10]. T1 height recovers to 75% of control at a TOFR of 0.25, and returns to 90% of control when the TOFR exceeds 0.9 in electromyographic records [8]. However, in acceleromyographic monitoring, T1 returns to less than 75% of control on average when the TOFR is 0.7. When the TOFR returns to 0.9, T1 is still less than 90% of control [11]. We confirmed a similar relationship between T1 and TOFR after reversal of neostigmine in acceleromyographic recordings (Fig. 2).
Results differ between mechanomyography and acceleromyography at approximately 50% twitch height maximally, with acceleromyography frequently overestimating the extent of the block [12]. The overestimated data from acceleromyographic monitoring should be normalized by baseline values to reliably detect residual paralysis [13]. Data normalization was not necessary in the present study, because TOFR and T1 recovery were recorded in a TOF response in each patient.
TOFR shows significantly more rapid recovery than T1 height after an optimum dose of sugammadex [4,5]. If the administered dose of sugammadex is small, the recovery of the TOFR to 0.9 appears significantly slower than recovery of T1 to 90% [5]. A TOFR of 0.9 may not guarantee an appropriate recovery of neuromuscular function if T1 is still less than 90% after sugammadex administration. We found that after sugammadex 2 mg/kg during rocuronium-induced moderate NMB, the T1 height recovered to 90% by 6 min after administration, although TOFR was already recovered to 0.9. Furthermore, since the recovered T1 was less than 80% until 2 min after administration, a TOFR of 1.0 may not be sufficient for the quick and appropriate recovery of neuromuscular relaxation (Table 2). If the inhalation and intravenous anesthetics are early stopped before the administration of sugammadex to reduce the recovery time, the patient may be in the awakening before complete recovery from muscle relaxation. At that time, the patient is likely to experience an unpleasant feeling due to residual paralysis, which causes a sudden and harsh response in the recovery process. We suspect this symptom is happened due to still depression of a T1 compared with full recovery of a TOFR. Maintenance of sevoflurane until administration of sugammadex allows the time for washing out of the inhalation anesthetic to awaken.
The reason for the difference between TOFR and first twitch after reversal with sugammadex is unclear, but we suspect that sugammadex results in a drastic reduction of the effective site concentration of the unbound relaxant due to formation of a stable complex with rocuronium in the plasma. Through a gradient of rocuronium molecules between the plasma and neuromuscular junction, NMBAs diffuse away from the nicotinic receptor, providing rapid recovery from NMB and prompt recovery of TOFR. However, in contrast to the TOFR, the process does not promote reestablishment of T1 with the same proportion and speed, i.e., muscle receptors are still blocked by rocuronium in the short term [14].
The previous reports [4,5] compared simply the time of a T1 and TOFR to 0.9, not described the specific pattern of recovery percent after sugammadex during rocuronium-induced deep NMB. In the present study, however, all neuromuscular monitoring data were saved in a personal computer using TOF-Watch SX Monitor software. So the detailed relationship between a TOFR and T1 can be analyzed following the time after sugammadex administration.
This study contains some limitations. The present results are unique to patients undergoing moderate rocuronium-induced NMB, and may not be generalizable to patients undergoing deep or pronounced rocuronium-induced NMB. The current results are difficult to actually verify in patients during recovery from anesthesia, because it is not possible to distinguish the impact of anesthetics and opioids from those of NMBAs and reversal agents. Maintenance of sevoflurane delays the recovery from rocuronium compared with intravenous anesthesia. Even though this trial has been implemented in the same environment in each group, sevoflurane may have affected recovery from rocuronium.
In conclusion, both TOFR and T1 should be considered when confirming adequate recovery in the 6 min after reversal with sugammadex administration during rocuronium-induced moderate NMB, if you have not conducted a separate T1 monitoring.
References
1. Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, et al. Intraoperative acceleromyographic monitoring reduces the risk of residual neuromuscular blockade and adverse respiratory events in the postanesthesia care unit. Anesthesiology 2008; 109: 389-398. PMID: 18719436.


2. van Santen G, Fidler V, Houwertjes MC, Top WM, Wierda JM. The effect of single twitch and train-of-four stimulation on twitch forces during stable neuromuscular block. J Clin Monit Comput 2000; 16: 529-533. PMID: 12580212.


3. Fuchs-Buder T, Claudius C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Mogensen J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand 2007; 51: 789-808. PMID: 17635389.


4. Suzuki T. A train-of-four ratio of 0.9 may not certify adequate recovery after sugammadex. Acta Anaesthesiol Scand 2011; 55: 368-369. PMID: 21288221.


5. Staals LM, Driessen JJ, Van Egmond J, De Boer HD, Klimek M, Flockton EA, et al. Train-of-four ratio recovery often precedes twitch recovery when neuromuscular block is reversed by sugammadex. Acta Anaesthesiol Scand 2011; 55: 700-707. PMID: 21574968.


6. Kopman AF, Yee PS, Neuman GG. Relationship of the train-of-four fade ratio to clinical signs and symptoms of residual paralysis in awake volunteers. Anesthesiology 1997; 86: 765-771. PMID: 9105219.


7. Sundman E, Witt H, Olsson R, Ekberg O, Kuylenstierna R, Eriksson LI. The incidence and mechanisms of pharyngeal and upper esophageal dysfunction in partially paralyzed humans: pharyngeal videoradiography and simultaneous manometry after atracurium. Anesthesiology 2000; 92: 977-984. PMID: 10754616.


8. Power SJ, Jones RM. Relationship between single twitch depression and train-of-four fade: influence of relaxant dose during onset and spontaneous offset of neuromuscular blockade. Anesth Analg 1987; 66: 633-636. PMID: 2886077.


9. Waud BE, Waud DR. The relation between the response to "train-of-four" stimulation and receptor occlusion during competitive neuromuscular block. Anesthesiology 1972; 37: 413-416. PMID: 5074722.


10. Lee CM. Train-of-4 quantitation of competitive neuromuscular block. Anesth Analg 1975; 54: 649-653. PMID: 1237253.


11. Kopman AF, Klewicka MM, Neuman GG. The relationship between acceleromyographic train-of-four fade and single twitch depression. Anesthesiology 2002; 96: 583-587. PMID: 11873031.


12. Harper NJ, Martlew R, Strang T, Wallace M. Monitoring neuromuscular block by acceleromyography: comparison of the Mini-Accelograph with the Myograph 2000. Br J Anaesth 1994; 72: 411-414. PMID: 8155441.



13. Suzuki T, Fukano N, Kitajima O, Saeki S, Ogawa S. Normalization of acceleromyographic train-of-four ratio by baseline value for detecting residual neuromuscular block. Br J Anaesth 2006; 96: 44-47. PMID: 16299046.



14. Sorgenfrei IF, Norrild K, Larsen PB, Stensballe J, Ostergaard D, Prins ME, et al. Reversal of rocuronium-induced neuromuscular block by the selective relaxant binding agent sugammadex: a dose-finding and safety study. Anesthesiology 2006; 104: 667-674. PMID: 16571960.


Fig. 2
Progress of the first twitch and the train-of-four ratio after administration of neostigmine during rocuronium-induced neuromuscular block. Neostigmine is administered at time 0 when the second twitch reappears in TOF stimulation.

Fig. 3
Progress of the first twitch and the train-of-four ratio after administration of sugammadex during rocuronium-induced neuromuscular block. Sugammadex is administered at time 0 when the second twitch reappears in TOF stimulation. *P < 0.001 versus first twitch. †P < 0.01 versus first twitch.