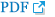Introduction
Neuromuscular blockade (NMB) is widely used during surgery to facilitate tracheal intubation and to minimize patient movement during the surgical procedure. After surgery, rapid reversal of the NMB is desirable to improve patient comfort and safety [
1], and to prevent post-operative complications such as hypoxia, weakness, and respiratory failure, which may increase patient morbidity [
2,
3].
Neostigmine, an acetylcholinesterase inhibitor, is commonly used in clinical practice in Korea to reverse NMB [
4]. Adverse effects associated with acetylcholinesterase inhibitors include bradycardia, bronchoconstriction, and increased gastric motility [
5]. Anticholinergic agents are usually administered in combination with acetylcholinesterase inhibitors to reduce these effects, but these agents are also associated with adverse effects such as blurred vision and tachycardia [
6].
Sugammadex, a selective relaxant-binding agent, rapidly and completely reverses the effects of the neuromuscular blocking agents rocuronium and vecuronium [
1,
7,
8]. It was approved in the European Union in 2008 for the reversal of moderate (reappearance of the second twitch of the train-of-four [TOF] response [T
2]; sugammadex 2.0 mg/kg) and deep (1-2 post-tetanic counts; sugammadex 4.0 mg/kg) NMB induced by rocuronium or vecuronium, and is currently approved in more than 70 countries worldwide. The present study investigated the use of sugammadex for reversing moderate NMB.
In Caucasian patients, sugammadex at 2.0 mg/kg has been demonstrated to provide significantly faster reversal of moderate NMB than neostigmine [
7]. In this pivotal study for this indication, the geometric mean time to recovery of the TOF ratio to 0.9 was 1.5 minutes with sugammadex compared to 18.6 minutes with neostigmine after each agent was administered at the reappearance of T
2 [
7]. To date, sugammadex has not been studied in Korean patients.
This was a local registration trial in Korea to evaluate and compare the efficacy and safety of sugammadex 2.0 mg/kg with neostigmine 50 µg/kg for reversal of moderate rocuronium-induced NMB in Korean patients. Moderate, rather than deep, NMB was chosen based on guidelines from the Korea Food and Drug Administration. A secondary objective of the study was to demonstrate similar recovery times as those observed in Caucasian patients based on data from a pivotal Phase III clinical trial of similar design conducted in Europe [
7].
Materials and Methods
This randomized, parallel-group, active-controlled, safety assessor-sblinded phase IV study (NCT01050543; sponsor protocol number P06101) was conducted at seven sites in the Republic of Korea. The study was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate Institutional Review Boards and regulatory agencies. All patients provided written informed consent before enrollment.
Adult patients were eligible for inclusion in the study provided they were American Society of Anesthesiologists Class 1, 2, or 3, scheduled for an elective surgical procedure under general anesthesia using rocuronium for tracheal intubation and maintenance of NMB, and required reversal of NMB. All patients were to be of Korean descent, born in Korea, never having emigrated out of Korea, and with a Korean home address. Exclusion criteria were any anatomical malformation that might cause difficult intubation; any patient transferred to the intensive care unit after surgery; neuromuscular disorders that could affect the NMB; significant renal or hepatic dysfunction; requirement of a pneumatic tourniquet during surgery; (family) history of malignant hyperthermia; allergy to opioids/opiates, cyclodextrins including sugammadex, muscle relaxants and their excipients, or other medications used during general anesthesia; administration of toremifene and/or fusidic acid within 24 hours of study drug administration (or plan to administer these drugs within 24 hours after study drug administration); any condition contraindicating neostigmine and/or glycopyrrolate; pregnant females; participation in a previous sugammadex study; participation in another clinical drug study within 30 days inclusive of signing consent for the current study; or a member of, or related to, the investigational staff or sponsor staff.
Eligible patients were randomized on a 1 : 1 basis to receive either sugammadex 2.0 mg/kg or neostigmine 50 µg/kg plus glycopyrrolate 10 µg/kg for NMB reversal. Anesthesia was induced with intravenous propofol and maintained with inhalational sevoflurane. Opioids were administered according to local practice when clinically required. Neuromuscular monitoring was carried out using continuous acceleromyography at the adductor pollicis muscle with the TOF-Watch SX® (Organon Ireland Ltd., a subsidiary of Merck and Co., Swords, Co. Dublin, Ireland). Following induction of anesthesia, the TOF-Watch SX® device was attached, stabilized, and calibrated.
Rocuronium 0.6 mg/kg was administered as a single bolus dose for intubation, and NMB was maintained with one or more doses of 0.1 to 0.2 mg/kg rocuronium as clinically required. After the last dose of rocuronium, at the reappearance of T2, a single intravenous (IV) dose of sugammadex 2.0 mg/kg or a single IV dose of neostigmine 50 µg/kg plus glycopyrrolate 10 µg/kg was administered to reverse the NMB.
Efficacy
The primary efficacy endpoint was the time from the start of administration of sugammadex or neostigmine to recovery of the TOF ratio to 0.9. Secondary endpoints included time to recovery of the TOF ratio to 0.7 and 0.8. Time to reappearance of T2 after the last dose of rocuronium was also assessed.
Bridging analysis
A key secondary objective of the study was to show that the recovery time from moderate NMB with sugammadex in Korean patients was comparable to that observed in Caucasian patients from the rocuronium group of a similar study [
7]. Specifically, we aimed to show that the geometric mean recovery time of the TOF ratio to 0.9 from administration of sugammadex was, with high confidence, less than 3 minutes.
Safety
Each patient was monitored for safety for up to 7 days after administration of the study drug. Safety variables comprised all reported adverse events (AEs, including serious AEs), coded using the Medical Dictionary for Regulatory Activities (MedDRA version 14.0), vital signs, physical examination, and clinical evidence of residual NMB and recurrence of NMB. A blinded safety assessor performed the safety assessments in the post-operative period and in the follow-up period. The anesthesiologist administering the anesthesia during the surgical procedure was not blinded to the randomized study drug, but was not allowed to reveal the assigned treatment group to the safety assessor.
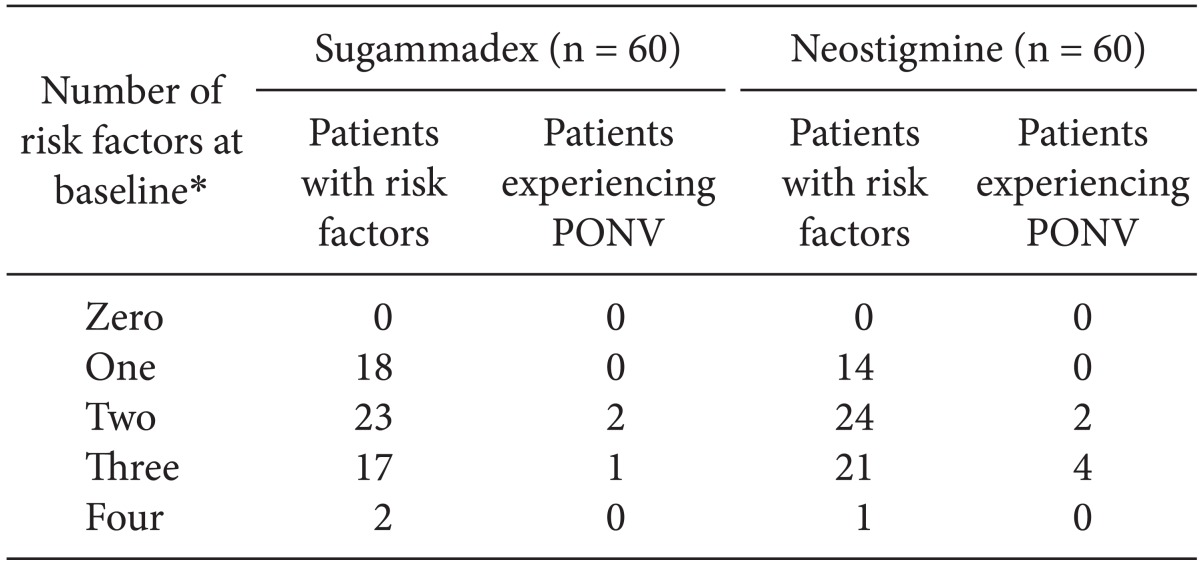
Risk factors for post-operative nausea and vomiting (PONV) [
9] were also assessed at baseline and used to assess the likelihood that any occurrences of PONV were associated with the study therapy. Subsequent incidence of PONV was determined from AEs coded with the MedDRA-preferred terms of nausea, procedural nausea, vomiting, and procedural vomiting.
Bridging analysis
Safety results observed for Korean subjects in the current study were compared retrospectively with those observed for Caucasian subjects in the rocuronium group of a similar study [
7].
Statistical analyses, populations, and sample size calculation
The primary efficacy endpoint was analyzed using a two-way analysis of variance (ANOVA) model that included the treatment groups and study site factors. As recovery times were expected to follow a lognormal distribution [
1], the response variable of the ANOVA model was the logarithm of the recovery time. A 95% confidence interval (CI) for the mean difference between the treatment groups was calculated on the log scale and transformed back onto the original scale, resulting in a CI for the ratio of the geometric mean recovery time after sugammadex dosing over the geometric mean recovery time after neostigmine dosing.
A hierarchical testing procedure was applied to cope with multiplicity due to performing two statistical tests related to the primary and secondary objectives of the study. First, the primary statistical comparison was performed at a significance level of 5%. Only if that test was successful (i.e., a statistically significant faster recovery time to a TOF ratio of 0.9 with sugammadex compared to neostigmine) was the secondary objective (equivalence with respect to time from sugammadex administration to recovery of the TOF ratio to 0.9 between Korean and Caucasian subjects) tested at a significance level of 5%. The hierarchical testing procedure followed the closed testing principle and maintained an overall error level of 5%.
The all subjects treated (AST) group was used to report demographic and baseline characteristics and safety data, and included all randomized patients who received a dose of study medication. The primary efficacy analysis was performed on the intention-to-treat (ITT) group, which included all patients who received randomized treatment and had at least one efficacy measurement. Data were imputed for patients with missing times to recovery of the TOF ratio to 0.7, 0.8, and 0.9 using a worst-case scenario for sugammadex and a best-case scenario for neostigmine. The per-protocol population comprised all subjects from the ITT group without any major protocol violation.
Based on a previous study of similar design, in which geometric mean times to recovery of the TOF ratio to 0.9 following reversal of rocuronium were approximately 1.5 minutes for sugammadex and 18.6 minutes for neostigmine [
7], and assuming that similar mean recovery times and variation would be observed in the Korean population in the present study, a sample size of 50 evaluable patients per treatment group was determined to provide sufficient power for the analyses. This would provide statistical confirmation of whether the geometric mean recovery time with sugammadex, with 95% confidence, is at least five times faster than the geometric mean time with neostigmine, and whether the geometric mean recovery time with sugammadex, with 95% confidence, is less than 3 minutes (i.e., not more than 1.5 minutes longer than the geometric mean recovery time of the Caucasian patients observed in the previous study [
7]). All statistical analyses were performed using the software package SAS® (SAS Institute, Cary, NC, USA; version 9.1 [Korean patients], version 8.2 [previous study in Caucasian patients]).
Safety data were analyzed using the AST population and summarized using descriptive statistics; no statistical tests were performed.
Results
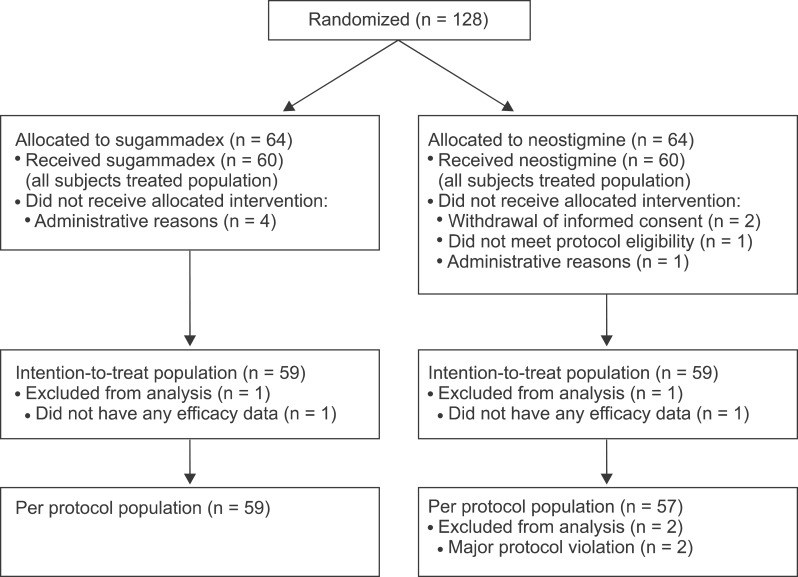
Overall, 128 patients were randomized in the study (
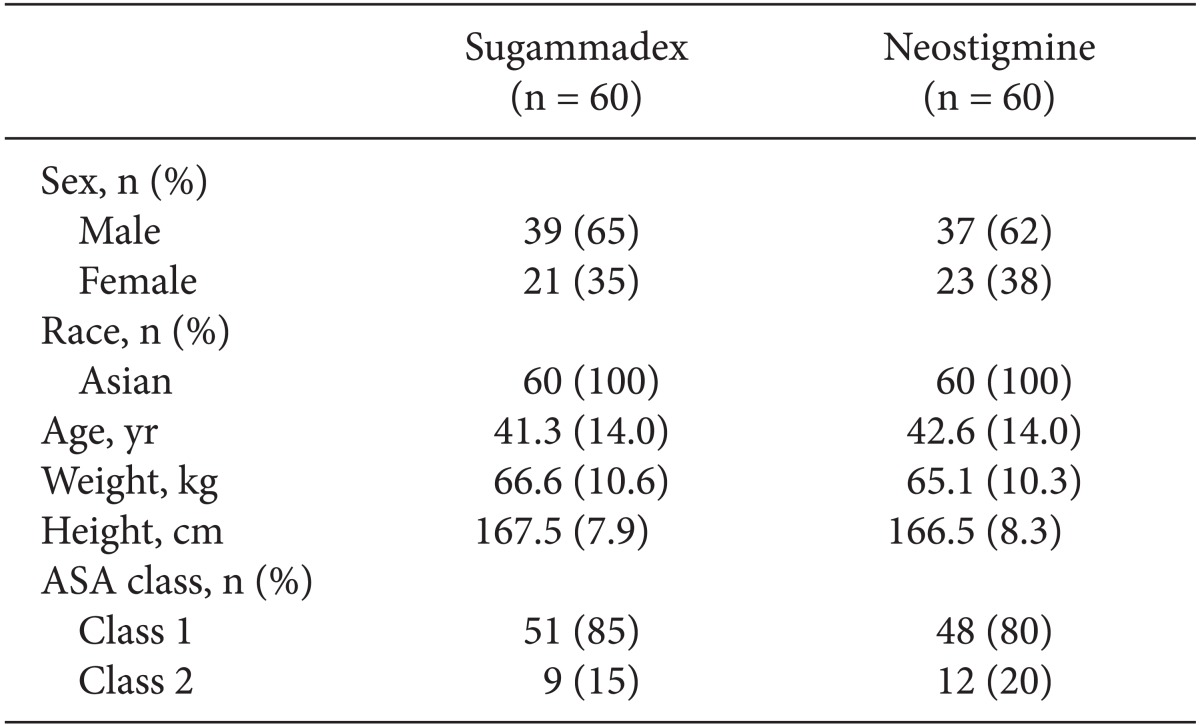
Fig. 1). The AST population comprised 60 patients in each treatment group and the ITT population consisted of 59 patients in each group. Patient demographics were well balanced between the two treatment groups (
Table 1). The types of elective surgical procedures performed were generally comparable between the two groups, with the most frequent procedures according to the Nordic Medico-Statistical Committee classification of surgical procedures being operations of the ear, nose, and larynx (48%), female genital organs (18%), and digestive system and spleen (17%).
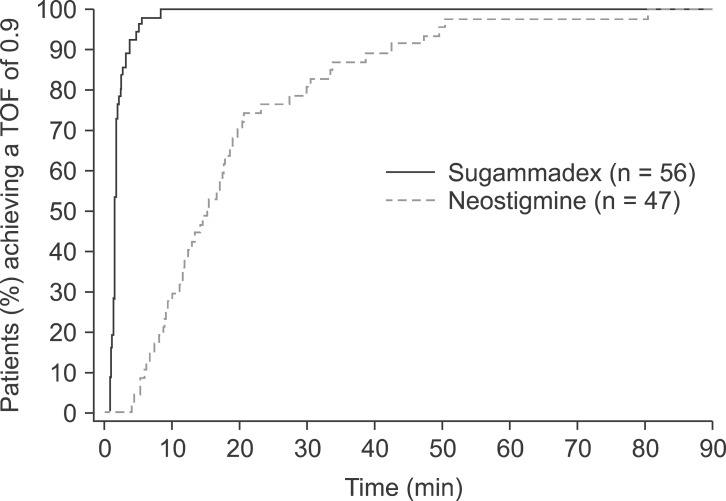
The geometric mean time to recovery of the TOF ratio to 0.9 was 1.8 minutes following administration of sugammadex 2.0 mg/kg compared with 14.8 minutes following administration of neostigmine 50 µg/kg (
Table 2). Based on statistical analysis of the logarithm of recovery times using a two-way ANOVA adjusted for trial site, recovery was estimated to be 8.1 times faster with sugammadex than with neostigmine (95% CI 6.8-9.6 times faster; P < 0.0001). Data were imputed for 3 patients in the sugammadex group and 11 patients in the neostigmine group. An analysis using only data from patients who had results available provided similar outcomes: for those patients with complete data (sugammadex, n = 56; neostigmine, n = 48), the geometric mean time to recovery of the TOF ratio to 0.9 was 1.7 minutes following administration of sugammadex 2.0 mg/kg compared with 15.3 minutes following administration of neostigmine 50 µg/kg.
Similar differences between the treatment groups were observed for recovery of the TOF ratio to 0.7 and 0.8: recovery times following sugammadex administration were 5.6 and 7.5 times faster, respectively, versus neostigmine (P < 0.0001 for both).
For each of the time-to-recovery endpoints (recovery of the TOF ratio to 0.7, 0.8, and 0.9), a statistically significant main effect of the trial site effect was observed (P < 0.0001), indicating that the geometric mean times, adjusted for treatment group, were not comparable across the seven trial sites. However, there was no statistically significant interaction between the trial site and treatment group for the time-to-recovery endpoints in the sensitivity analysis, indicating that the treatment effect of sugammadex versus neostigmine was homogenous across the trial sites.
Fig. 2 illustrates the cumulative percentage of patients for the time taken to recover to a TOF ratio of 0.9, based on the perprotocol populations with complete recovery data available. For most patients (89.3%; 50/56 patients) in the sugammadex group, the time to recovery of the TOF ratio to 0.9 was less than 3 minutes (
Fig. 2). By comparison, in the neostigmine group, it took 38.5 minutes before a similar proportion of patients (89.4%; 42/47 patients) had recovered to a TOF ratio of 0.9 and no patients recovered within 3 minutes. The geometric mean times to recovery of T
2 after the start of administration of the last dose of rocuronium were similar in both groups: 26.2 minutes in the sugammadex group and 29.0 minutes in the neostigmine group.
The geometric mean times to recovery of the TOF ratio to 0.9 for Korean and Caucasian patients following administration of sugammadex 2.0 mg/kg at the return of T
2 were comparable between the two patient groups (1.8 and 1.5 minutes, respectively;
Table 2). In both patient populations, the upper limit of the geometric mean 95% CI was less than the pre-specified criterion of 3 minutes (the upper 95% CIs were 2.0 and 1.7 minutes for Korean and Caucasian patients, respectively).
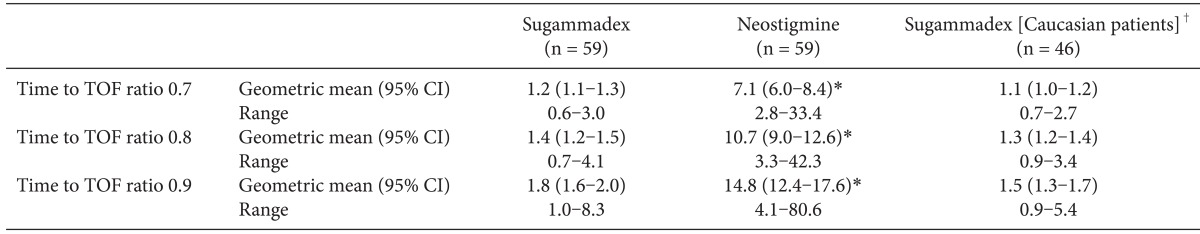
The percentage of patients who experienced an AE was similar between the groups, with a total of 98 AEs reported in 41 (68%) patients who received sugammadex, and a total of 117 AEs reported in 44 (73%) patients who received neostigmine (
Table 3). Most AEs were of mild or moderate intensity. The percentage of subjects reported to have experienced at least one non-serious AE of severe intensity was similar between the treatment groups. In the sugammadex group, two subjects (3%) experienced a severe headache, while in the neostigmine group three subjects (5%) experienced severe AEs: one subject experienced a severe headache and two subjects experienced severe incision site pain. Treatment-related AEs (i.e., those that were considered to be possibly or probably related to the study drug) were reported for four (7%) patients in the sugammadex treatment group, and six (10%) patients in the neostigmine treatment group. In the sugammadex group, these were cardiac anesthetic complication (bradycardia of moderate intensity, n = 1) and headache (n = 3); in the neostigmine group, they were headache (n = 2), nausea, recurrence of NMB, rash, and hypotension (n = 1).
Serious AEs were reported for two patients in each treatment group, all of which were considered unlikely to be related to the study drug. In the sugammadex group, these included a severe intestinal anastomosis in a 69-year-old male and a severe postoperative abscess in a 48-year-old female. In the neostigmine group, these were metastases to the bone (severe) in a 56-year-old male with maxillary sinus squamous cell carcinoma, and moderate dysuria in a 45-year-old female.
PONV was reported in three patients in the sugammadex group and six patients in the neostigmine group (
Table 4). All patients from both treatment groups who experienced PONV had at least two baseline PONV risk factors.
There were no clinically relevant differences between the treatment groups in mean systolic or diastolic blood pressures or heart rate.
No AEs that would be potentially indicative of inadequate reversal of NMB were reported for any sugammadex patients. In the neostigmine group, four patients (7%) reported AEs that were possibly indicative of inadequate reversal (mild amblyopia, mild asthenia and two cases of mild recurrence of NMB).
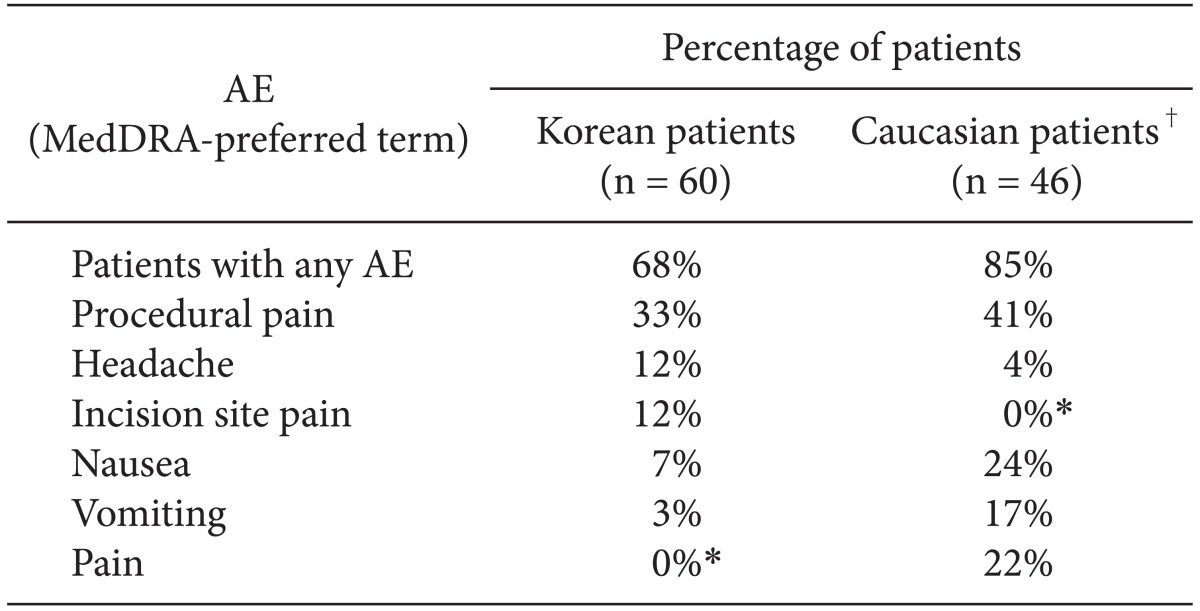
The most frequently occurring AEs (occurring in ≥ 10% of Korean or Caucasian patients who received sugammadex) are listed in
Table 5. A lower proportion of treatment-emergent AEs were reported for Korean patients (68% in the sugammadex group) than for Caucasian patients (85% in the sugammadex group) (
Table 5). Headache was reported more frequently in Korean patients than for Caucasian patients (12% and 4%, respectively), while nausea and vomiting were reported more frequently in Caucasian patients (24% and 17% vs. 7% and 3%, respectively, in Korean patients).
Discussion
To date, sugammadex has been extensively studied in Western patients living in Europe and the USA for the reversal of moderate and deep NMB [
1,
7,
8], but it is important to investigate it in other patient populations, particularly because ethnicity and geographic location may affect the potency and duration of action of many drugs [
10-
13].
This was the first randomized, active-controlled trial of sugammadex in Korean patients, comparing recovery from rocuronium-induced NMB between sugammadex 2.0 mg/kg and neostigmine 50 µg/kg administered at the reappearance of T
2 (moderate blockade). In Korean patients, the recovery to a TOF ratio of 0.9 was approximately 8.1 times faster after administration of sugammadex compared with neostigmine, when administered at the reappearance of T
2 (1.8 minutes compared with 14.8 minutes, P < 0.0001). Geometric mean times to recovery of the TOF ratio to 0.9 in Korean patients were comparable to those observed in a previous, similarly designed pivotal study in Caucasian patients (1.5 minutes and 18.6 minutes in the sugammadex and neostigmine groups, respectively) [
7].
A key secondary objective of this study was to show that the geometric mean time to recovery of the TOF ratio following administration of sugammadex was, with 95% confidence, less than 3 minutes. The data confirmed this, with the upper limit of the 95% CI for geometric mean time to recovery at 2.0 minutes.
Treatment with sugammadex in Korean patients was generally well tolerated. A similar percentage of patients in each treatment group experienced at least 1 AE (68% in the sugammadex group and 73% in the neostigmine group). Serious AEs were reported for two patients in each group, while PONV was reported in three patients in the sugammadex group and six patients in the neostigmine group. Importantly, there was no evidence of residual or recurrence of NMB in patients who received sugammadex in the present study, but there was evidence of inadequate NMB reversal (based on AE reporting) in 7% of patients who received neostigmine.
Compared to Caucasian patients from the previous study [
7], a lower proportion of AEs was found in Korean patients (85% and 68%, for Caucasian and Korean patients, respectively). The overall safety profile was similar for both patient populations.
In conclusion, sugammadex 2 mg/kg provided rapid and complete reversal of moderate rocuronium-induced NMB in Korean patients, and the time to recovery of the TOF ratio to 0.9 was significantly (~8.1 times) faster with sugammadex than with neostigmine (P < 0.0001). The overall efficacy and safety profiles of sugammadex were similar to those previously observed for Caucasian patients in a comparable pivotal study that examined reversal of moderate NMB.
Acknowledgments
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. Assistance with the design and execution of the study was provided by Joris de Bie (Merck Sharp & Dohme Corp., White-house Station, NJ, USA). Additional statistical support was provided by Henk Rietbergen (MSD, Oss, The Netherlands). Medical writing support was provided by Neil Venn from Prime Medica (Knutsford, Cheshire, UK) during the preparation of this manuscript, supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA. Responsibility for opinions, conclusions, and interpretation of the data lies with the authors.
Disclosures: Tiffany Woo and Phillip Phiri are employees of Merck Sharp & Dohme Corp., Whitehouse Station, NJ.
References
1. Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology 2008; 109: 816-824. PMID:
18946293.


2. Murphy GS. Residual neuromuscular blockade: incidence, assessment, and relevance in the postoperative period. Minerva Anestesiol 2006; 72: 97-109. PMID:
16493386.

3. Plaud B, Debaene B, Donati F, Marty J. Residual paralysis after emergence from anesthesia. Anesthesiology 2010; 112: 1013-1022. PMID:
20234315.


4. Kang SK, Choi YR, Chung CD. The effect of atropine-neostigmine and glycopyrrolate-neostigmine mixture on heart rate. Korean J Anesthesiol 1987; 20: 166-171.

5. Booij LH, de Boer HD, van Egmond J. Reversal agents for nondepolarizing neuromuscular blockade: reasons for and development of a new concept. Semin Anesth Periop Med Pain 2002; 32: 92-98.

6. Feinberg M. The problems of anticholinergic adverse effects in older patients. Drugs Aging 1993; 3: 335-348. PMID:
8369593.


7. Blobner M, Eriksson LI, Scholz J, Motsch J, Della Rocca G, Prins ME. Reversal of rocuronium-induced neuromuscular blockade with sugammadex compared with neostigmine during sevoflurane anaesthesia: results of a randomized, controlled trial. Eur J Anaesthesiol 2010; 27: 874-881. PMID:
20683334.


9. Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. Simplified risks score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999; 91: 693-700. PMID:
10485781.


10. Dahaba AA, Perelman SI, Moskowitz DM, Bennett HL, Shander A, Xiao Z, et al. Geographic regional differences in rocuronium bromide dose-response relation and time course of action: an overlooked factor in determining recommended dosage. Anesthesiology 2006; 104: 950-953. PMID:
16645446.


11. Collins LM, Bevan JC, Bevan DR, Villar GCP, Kahwaji R, Smith MF, et al. The prolonged duration of rocuronium in Chinese patients. Anesth Analg 2000; 91: 1526-1530. PMID:
11094012.


12. Fiset P, Donati F, Balendran P, Meistelman C, Lira E, Bevan DR. Vecuronium is more potent in Montreal than in Paris. Can J Anaesth 1991; 38: 717-721. PMID:
1680573.


13. Katz RL, Norman J, Seed RF, Conrad L. A comparison of the effects of suxamethonium and tubocurarine in patients in London and New York. Br J Anaesth 1969; 41: 1041-1047. PMID:
4312426.