Reduced side effects and improved pain management by continuous ketorolac infusion with patient-controlled fentanyl injection compared with single fentanyl administration in pelviscopic gynecologic surgery: a randomized, double-blind, controlled study
Article information
Abstract
Background
A combination of opioids and adjunctive drugs can be used for intravenous patient-controlled analgesia (PCA) to minimize opioid-related side effects. We investigated whether two different analgesics administered separately via a dual-chamber PCA have fewer side effects with adequate analgesia than a single fentanyl PCA in gynecologic pelviscopic surgery.
Methods
This prospective, double-blind, randomized, and controlled study included 68 patients who underwent pelviscopic gynecological surgery. Patients were allocated to either the dual (ketorolac and fentanyl delivered by a dual-chamber PCA) or the single (fentanyl alone) group. Postoperative nausea and vomiting (PONV) and analgesic quality were compared between the two groups at 2, 6, 12, and 24 h postoperatively.
Results
The dual group showed a significantly lower incidence of PONV during postoperative 2–6 h (P = 0.011) and 6–12 h (P = 0.009). Finally, only two patients (5.7%) in the dual group and 18 (54.5%) in the single group experienced PONV during the entire postoperative 24 h and could not maintain intravenous PCA (odds ratio: 0.056, 95% CI [0.007, 0.229], P < 0.001). Despite the administration of less fentanyl via intravenous PCA during the postoperative 24 h in the dual group than in the single group (66.0 ± 77.8 vs. 383.6 ± 70.1 μg, P < 0.001), postoperative pain had no significant intergroup difference.
Conclusions
Two different analgesics, continuous ketorolac and intermittent fentanyl bolus, administered via dual-chamber intravenous PCA, showed fewer side effects with adequate analgesia than conventional intravenous fentanyl PCA in gynecologic patients undergoing pelviscopic surgery.
Introduction
Patient-controlled analgesia (PCA) is a method that allows patients to self-manage pain using a programmable infusion pump. The analgesic drug is placed in a specially designed PCA device in advance, and a portion of it is continuously infused with an additional bolus dose as required. Although opioids are preferentially used for intravenous PCA [1,2], they have various side effects such as nausea, vomiting, dizziness, sedation, and respiratory depression [3,4]. These side effects are major causes for the unexpected early discontinuation of intravenous PCA.
To minimize opioid-related side effects, a combination of opioids with adjunctive medications, such as propacetamol [5], ketorolac [6], nefopam [7], and dexmedetomidine [8], is used. The existing intravenous PCA device is a single-chamber pump. Thus, adjunctive medication should be administered using an additional infusion pump or intermittent bolus injection. Mixing and storing two or more drugs in a single-chamber PCA cannot guarantee their physicochemical and microbiological stability [9]. Meanwhile, a novel dual-chamber PCA device (Bellomic DUO®; Cebika) has been launched that consists of one continuous flow chamber with another chamber having a bolus function.
We hypothesized that two different analgesics, ketorolac and fentanyl, administered separately via a dual-chamber PCA may have fewer side effects with adequate analgesia than those associated with conventional intravenous single-fentanyl PCA. Thus, the accompanying side effects and analgesic quality were evaluated in gynecological patients undergoing pelviscopic surgery.
Materials and Methods
Study design
The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (No. B-2110-716-003). This was a prospective randomized controlled trial conducted at our hospital in accordance with the principles of the Declaration of Helsinki, 2013. After obtaining written informed consent, patients were recruited for the study.
Study participants
Patients aged 20–65 years who were scheduled for elective laparoscopic gynecologic surgery under general anesthesia were enrolled in this study. According to the analgesic combination, patients were randomly assigned in a 1:1 allocation ratio to two parallel groups: the dual or single group. Block randomization with a block size of six was used to divide the participants into groups. One investigator was aware of the group arrangement and was involved in the preparation of the intravenous PCA. In addition, neither the participants nor the investigators knew to which group each participant was assigned. The exclusion criteria included an American Society of Anesthesiologists physical status classification III-V, pregnancy, side effects of opioids or hypersensitivity to aspirin, ketorolac, or other nonsteroidal anti-inflammatory drugs (NSAIDs), body mass index > 35 kg/m2 or < 18 kg/m2, alcohol or drug dependency, peptic ulcer or gastrointestinal bleeding, cerebrovascular hemorrhage, increased intracranial pressure, bronchial asthma or bronchospasm, severe respiratory depression, moderate to severe renal impairment or dehydration, nasal polyp, angioedema, a history of convulsive disease, patients for whom the use of neuromuscular blocking agents is contraindicated, or use of monoamine oxidase inhibitors.
General anesthesia
Noninvasive blood pressure, electrocardiography, pulse oximetry, and bispectral index were measured upon arrival at the operating room. With oxygen supplementation via an anesthetic face mask, anesthesia was induced with 1 mg/kg of propofol and target-controlled infusion of remifentanil at 3.0 ng/ml of the effect site concentration. After the loss of consciousness, 0.6 mg/kg of rocuronium was injected and tracheal intubation was performed. Anesthesia was maintained and adjusted using desflurane and remifentanil according to the bispectral indexTM (Medtronic) and hemodynamic changes, respectively. At the end of the surgery, intravenous PCA was connected following 0.3 mg of ramosetron. After confirming recovery of consciousness and spontaneous breathing, extubation was performed and patients were transferred to the post-anesthesia care unit (PACU). Patients were discharged from PACU when the post-anesthesia recovery score composed of vital signs, activity, postoperative nausea and vomiting (PONV), pain, and surgical bleeding, became 10.
Intravenous PCA device and analgesic regimen
A dual-chamber PCA device with continuous and selector chambers is commonly used in both groups. The elastomeric pump of the continuous chamber has a drug to be infused at a 2 ml/h of fixed flow rate and the selector chamber has the function of injecting a bolus of 1 ml (10 min of lock-out period) as required without basal infusion.
In the dual group, the continuous chamber of the dual-chamber PCA device contained 180 mg of ketorolac with 94 ml of normal saline for a total volume of 100 ml. The single group received 700 μg of fentanyl with 86 ml of normal saline. The selector chamber in both groups contained 200 μg of fentanyl and 16 ml of normal saline to a total volume of 20 ml.
Intravenous PCA was discontinued if the patient experienced persistent vomiting or nausea and no longer wished to use it.
For rescue analgesia in the PACU, 25 μg of fentanyl was additionally administered if the numerical rating scale (NRS) for postoperative pain was 3 to 5 and 50 μg of fentanyl when the NRS for postoperative pain was 6 or higher. If a patient complained of pain with an NRS score of 4 or higher in the ward, 400 mg of ibuprofen was administered as the first rescue analgesic drug at least 4 h apart. Nevertheless, if the NRS score was still ≥ 4, 100 mg of tramadol was administered additionally.
Outcome variables
The primary outcome was the incidence of PONV that was evaluated during the PACU stay, 0–2 h, 2–6 h, 6–12 h, and 12–24 h. PONV severity (nausea, retching, vomiting) and the use of rescue antiemetics were evaluated together. Nausea was defined as a subjectively unpleasant sensation with an awareness of the urge to vomit. Retching was defined as labored, spasmodic contraction of the respiratory muscles without expulsion of the gastric contents. Vomiting was defined as the expulsion of the gastric contents. The secondary outcomes were the NRS score for postoperative pain, use of rescue analgesics, and reason for discontinuation of intravenous PCA.
Statistical analysis
We estimated a priori that 32 patients in each group would be sufficient to detect a decrease in PONV incidence from 40% to 10% in the single group versus the dual group (power of 80% and a risk of 0.05 for type I error). Based on the assumption of an overall rate of loss to follow-up of 10%, 36 patients per group were required.
The normal distribution of continuous variables was evaluated using the Shapiro-Wilk test. Normally distributed continuous variables are presented as mean (standard deviation [SD]), and if the distribution is not normal, the median (interquartile range) is presented. Incidence is presented as a number (%) with odds ratio (OR) and 95% confidence interval (CI). Categorical variables were compared using the χ2 test or Fisher’s exact test, whereas continuous variables were compared using the Student’s t-test or Mann-Whitney U test. Postoperative NRS scores for pain were analyzed using repeated-measures ANOVA. When the postoperative NRS score for pain showed a significant intergroup difference, it was compared using the Mann-Whitney U test at each time frame. The intention-to-treat population included patients who were initially randomized to each group. The full analysis set (FAS) was defined as the remaining population, after excluding patients from the intention-to-treat population who did not receive the designated intravenous PCA. All statistical analyses were performed using the R Statistical Software version 4.2.1 (Foundation for Statistical Computing). Values were considered statistically significant at two-sided P < 0.05.
Results
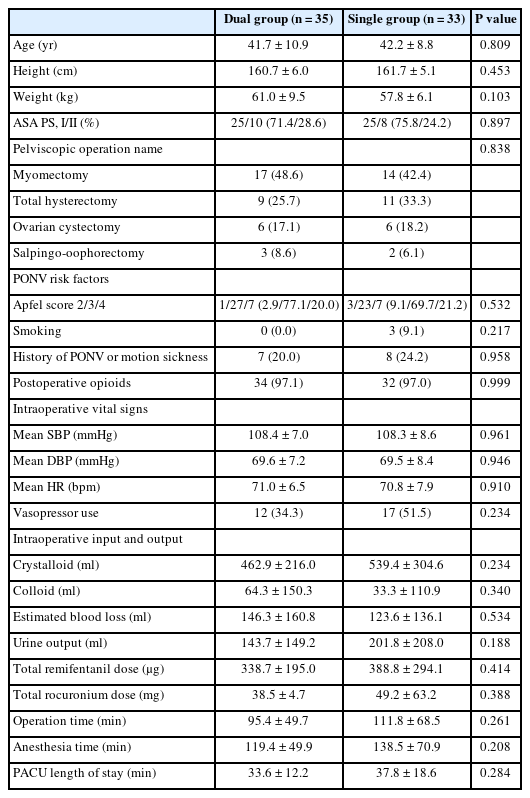
A total of 72 patients were enrolled from June 2022 to August 2022 and four patients were excluded because the intravenous PCA analgesics were configured differently from the protocol (Fig. 1). The characteristics of the patients, surgery, and anesthesia are summarized in Table 1, and they were comparable between the two groups.
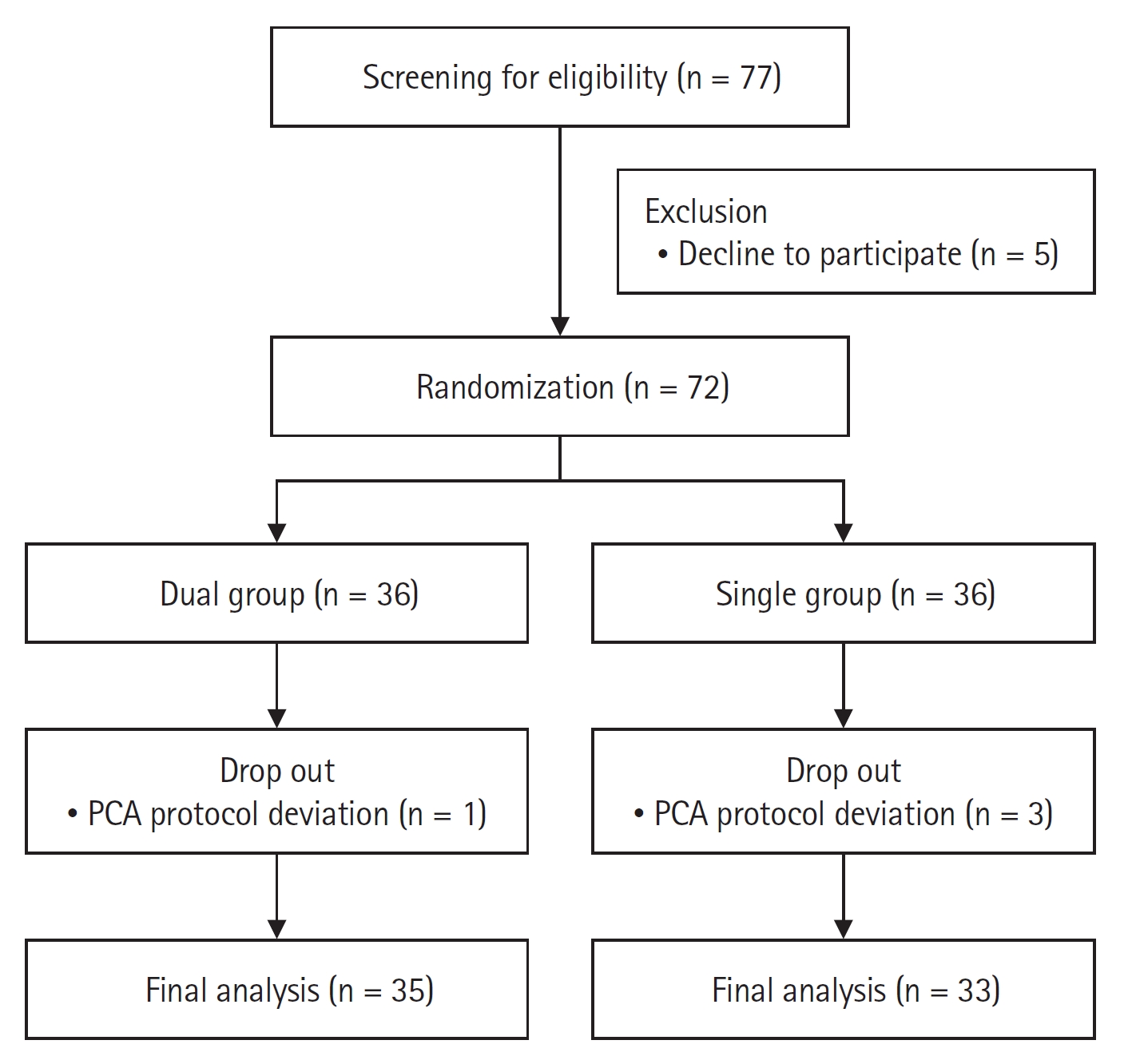
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the patients. Dual group: ketorolac and fentanyl delivered by a dual-chamber PCA, Single group: fentanyl alone delievered by a PCA. PCA: patientcontrolled analgesia.
The incidence of PONV did not significantly differ between the two groups at the PACU (P = 0.444) and until postoperative 2 h (P = 0.378). However, the dual group showed a significantly lower incidence of PONV during postoperative 2–6 h (P = 0.011) and 6–12 h (P = 0.009; Fig. 2 and Supplementary Table 1) postoperatively. Finally, only two patients (5.7%) in the dual group and 18 (54.5%) in the single group experienced PONV during the entire postoperative 24 h and could not maintain intravenous PCA (OR: 0.056, 95% CI [0.007, 0.229], P < 0.001).
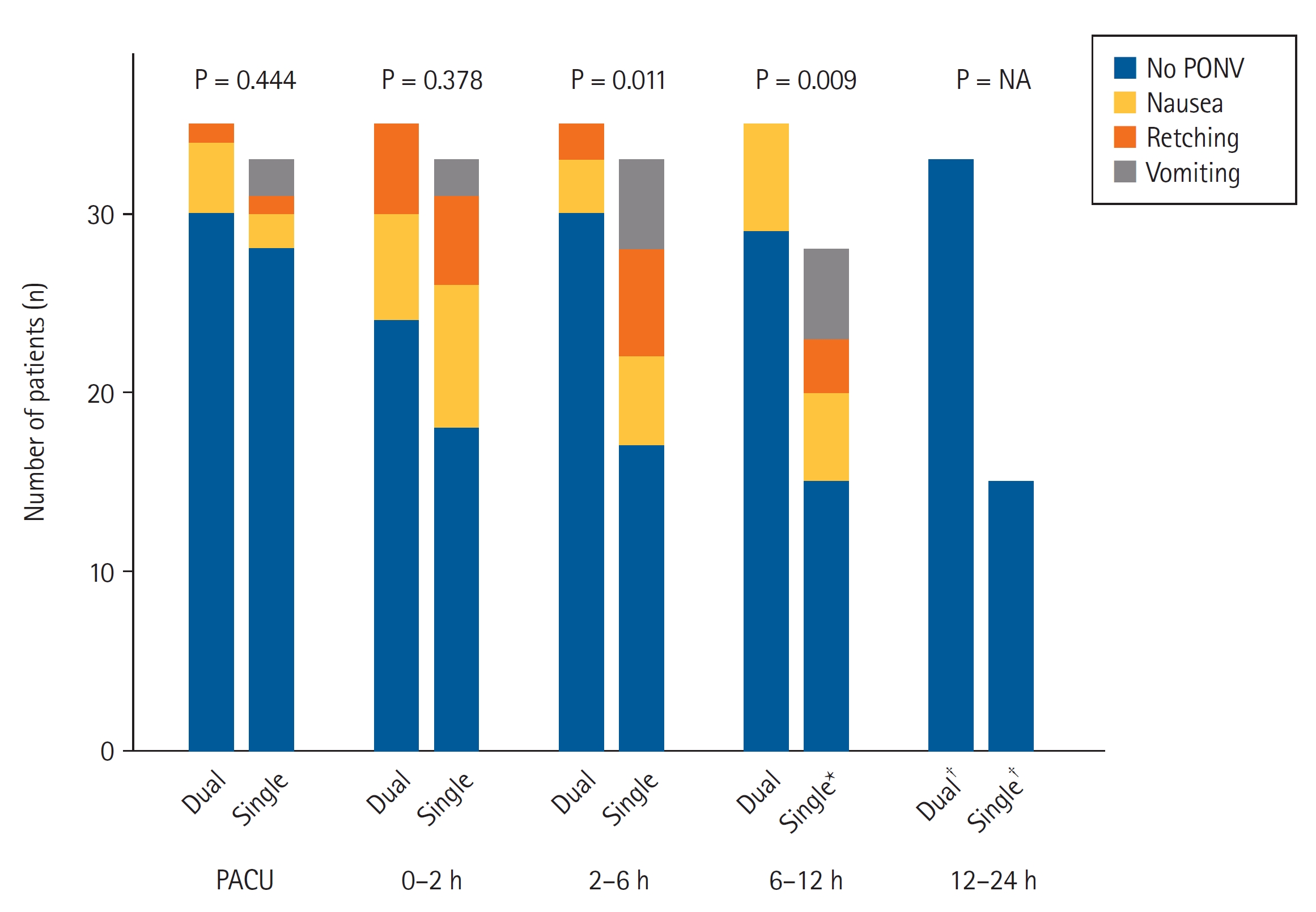
The incidence distribution of PONV in both groups. Dual group: ketorolac and fentanyl delivered by a dual-chamber PCA, Single group: fentanyl alone delievered by a PCA. PACU: post-anesthesia care unit, PONV: postoperative nausea and vomiting, PCA: patient-controlled analgesia, NA: not available. *Five patients in the single group discontinued the intravenous PCA at postoperative 2–6 h. †Two patients in the dual group and 13 patients in the single group discontinued the intravenous PCA at postoperative 6–12 h.
Postoperative NRS scores for pain showed no significant intergroup differences. It decreased over time, and significant differences were found at postoperative 2 h compared to the PACU values in each group (Fig. 3). Rescue fentanyl doses at PACU were similar between the two groups (90.7 ± 39.8 and 82.6 ± 34.5 μg in the dual and the single group, respectively; P = 0.372); however, less fentanyl was administered via intravenous PCA during the postoperative period in the dual group than in the single group (66.0 ± 77.8 vs. 383.6 ± 70.1 μg, P < 0.001). The proportion of patients who required rescue analgesics in the ward was similar between the two groups (Table 2). Tramadol was additionally administered to 12 (34.3%) and 11 patients (33.3%) in the dual and single group, respectively, at postoperative 0–2 h (P = 0.934).
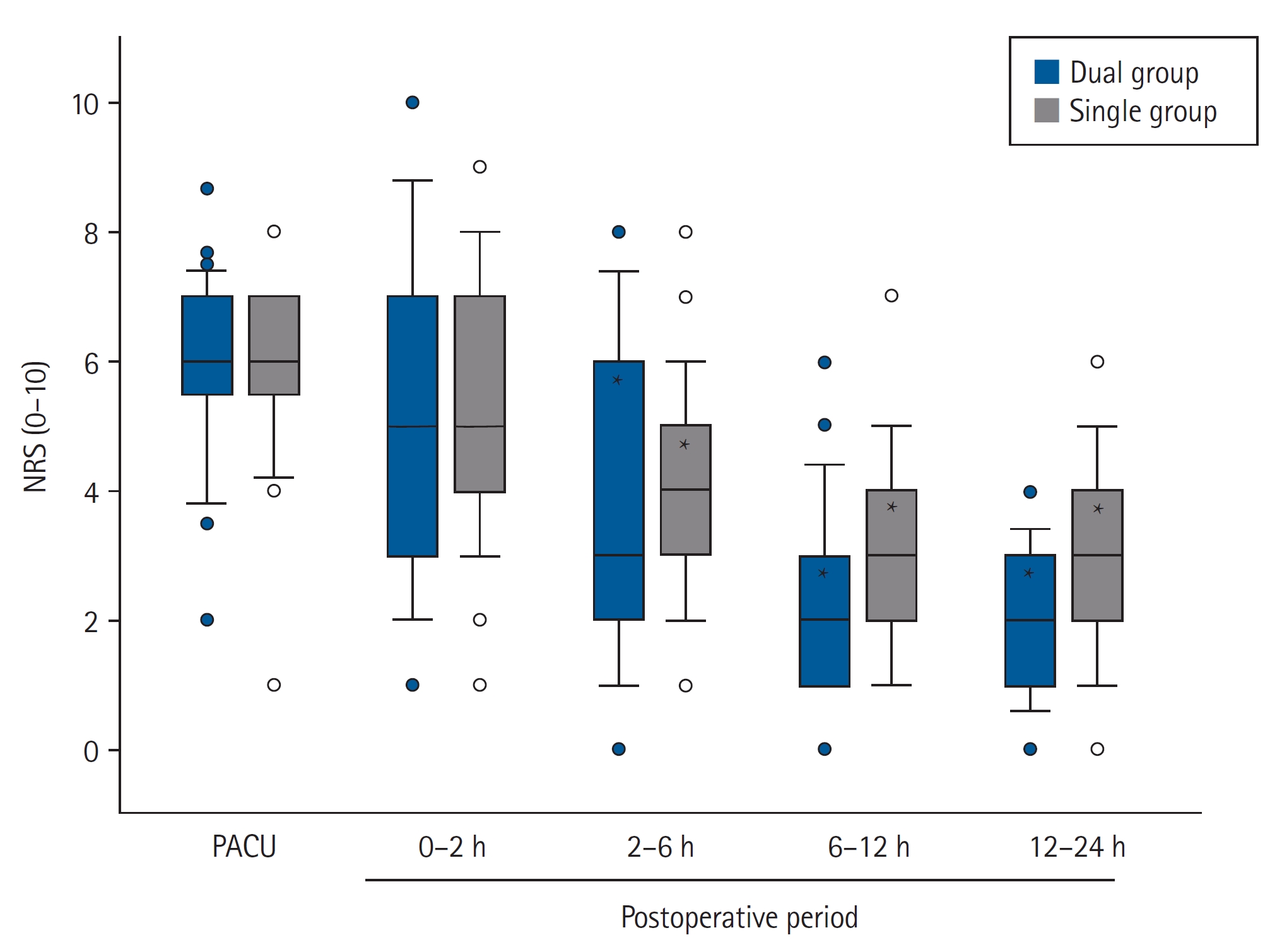
Postoperative NRS for pain in both groups. Dual group: ketorolac and fentanyl delivered by a dual-chamber PCA, Single group: fentanyl alone delievered by a PCA. PACU: post-anesthesia care unit, NRS: numerical rating scale. *P < 0.001 vs. NRS at PACU of each group.
Discussion
This study found that two different analgesics, ketorolac and fentanyl, could be administered safely using a newly designed dual-chamber intravenous PCA that was able to significantly reduce postoperative PONV while providing adequate pain control. The strength of this study is that it achieved multimodal analgesia with ketorolac and fentanyl via the dual-chamber intravenous PCA and evaluated its efficacy on postoperative analgesia and side effects compared to conventional opioid PCA. Oh et al. [10] used the same PCA device for postoperative analgesia and demonstrated the effect of the dual administration of ketorolac and fentanyl. The difference was that there was no basal infusion of opioids in our dual group that was administered only when the patient required them. The selective use of opioids in intravenous PCA in combination with ketorolac resulted in significantly reduced opioid-related side effects and improved analgesic efficacy.
In our study, the amount of fentanyl administered via intravenous PCA was reduced by one-sixth that is consistent with previous studies reporting that intranasal or intravenous ketorolac administration reduced opioid consumption in patients on a PCA morphine pump [11,12]. With this reduced opioid consumption, patients in the dual group experienced fewer opioid-related side effects, particularly PONV, without compromising the analgesic quality. The value of multimodal analgesia with NSAID and opioids for fewer postoperative adverse effects and better analgesic quality is clinically significant, as it is reportedly associated with shorter hospital stays and improved recovery [13–17].
Multimodal analgesia is an important method for managing postoperative pain to avoid excessive opioid consumption and its adverse effects [18]. The rationale for multimodal analgesia is to achieve sufficient analgesia by the additive or synergistic effects of different combined classes of analgesics that act via different mechanisms [15]. NSAIDs may be used to reduce postoperative opioid consumption and the incidence of opioid-related adverse events [12,19]; however, these studies evaluated the effect of perioperative single-dose intravenous NSAID administration on postoperative outcomes. NSAIDs can be used as the sole analgesic in minor surgeries. However, breakthrough pain should be managed separately with more potent opioids, limiting the widespread use of intravenous PCA composed of NSAIDs.
Jung et al. [4] previously reported that basal infusion of fentanyl-based intravenous PCA increased fentanyl consumption with more side effects; however, no benefit was observed in reducing pain intensity. The single group in our study also used fentanyl-based intravenous PCA, including basal infusion, and the results were similar to those of Jung et al. [4]. The quality of postoperative analgesia did not improve even when more fentanyl was administered to the single group via intravenous PCA than to the dual group. In addition, approximately 55% of the patients in the single group discontinued intravenous PCA; thus, their postoperative pain management might have been inappropriate.
Interestingly, the mean NRS scores for pain were significantly reduced at postoperative 2 h in both groups. In addition, the requirement for rescue analgesic drugs did not differ between the two groups. This means that the demand for analgesics is lower than expected for less-invasive pelviscopic operations. Controlling breakthrough pain with an opioid while administering ketorolac as a basal continuous infusion can be an appropriate intravenous PCA option in gynecologic pelviscopic surgery.
The pH of mixtures of two or more drugs decreases over time, and the concentration of ketorolac decreases significantly [9]. In addition, even if the stability of drugs in vitro is confirmed, it does not ensure pharmacodynamic and pharmacokinetic safety after administration to the patient’s body [9]. The chemical and microbiological stabilities of the various analgesic drug mixtures were evaluated according to their clinical combinations. Some studies have demonstrated the stability and compatibility of mixed drugs for a considerable period [20,21], whereas others have yet to draw firm conclusions because the results may be affected by the type or condition of the mixed drugs [22,23]. In this study, dual-chamber PCA enabled ketorolac to be infused as a basal analgesic drug, and fentanyl was administered as a bolus injection for breakthrough pain. Thus, the aforementioned safety issues can be resolved.
This study had several limitations. First, it was conducted at a single tertiary university hospital, and all patients were female who underwent pelviscopic gynecologic surgery that might have contributed to a selection bias. Second, the majority of our patients in both groups exhibited Apfel scores of 3 and 4; thus, the combined administration of ketorolac and fentanyl via dual-chamber intravenous PCA may not have significant effects in patients with fewer risk factors for PONV. Third, the direct effect of the dual-chamber NSAID and fentanyl PCA on hospital stay and quality of recovery was not assessed, as this study primarily focused on the immediate postoperative outcomes. Finally, all data were analyzed in the FAS population, except the incidence distribution of PONV (Fig. 2) that was analyzed only in patients who had used intravenous PCA at a designated period. Patients who discontinued intravenous PCA owing to PONV were excluded from the follow-up period. This caused the post-hoc power to decrease to 71.6% at postoperative 6–12 h. However, the final PONV incidence and intravenous PCA discontinuation rates over the entire observation period included all enrolled patients and met our hypothesis.
In conclusion, the two different analgesics, continuous ketorolac and intermittent fentanyl bolus, administered via a dual-chamber intravenous PCA, showed fewer side effects with adequate analgesia compared to those associated with conventional intravenous fentanyl PCA in gynecologic patients undergoing pelviscopic surgery.
Notes
Funding
This study was supported from the HB medical Research Fund.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Insun Park (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Visualization; Writing – original draft)
Seukyoung Hong (Data curation)
Su Yeon Kim (Data curation; Formal analysis)
Jung-Won Hwang (Conceptualization; Project administration; Writing – review & editing)
Sang-Hwan Do (Conceptualization; Supervision; Validation; Writing – review & editing)
Hyo-Seok Na (Conceptualization; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – review & editing)
Supplementary Material
Incidence of PONV.