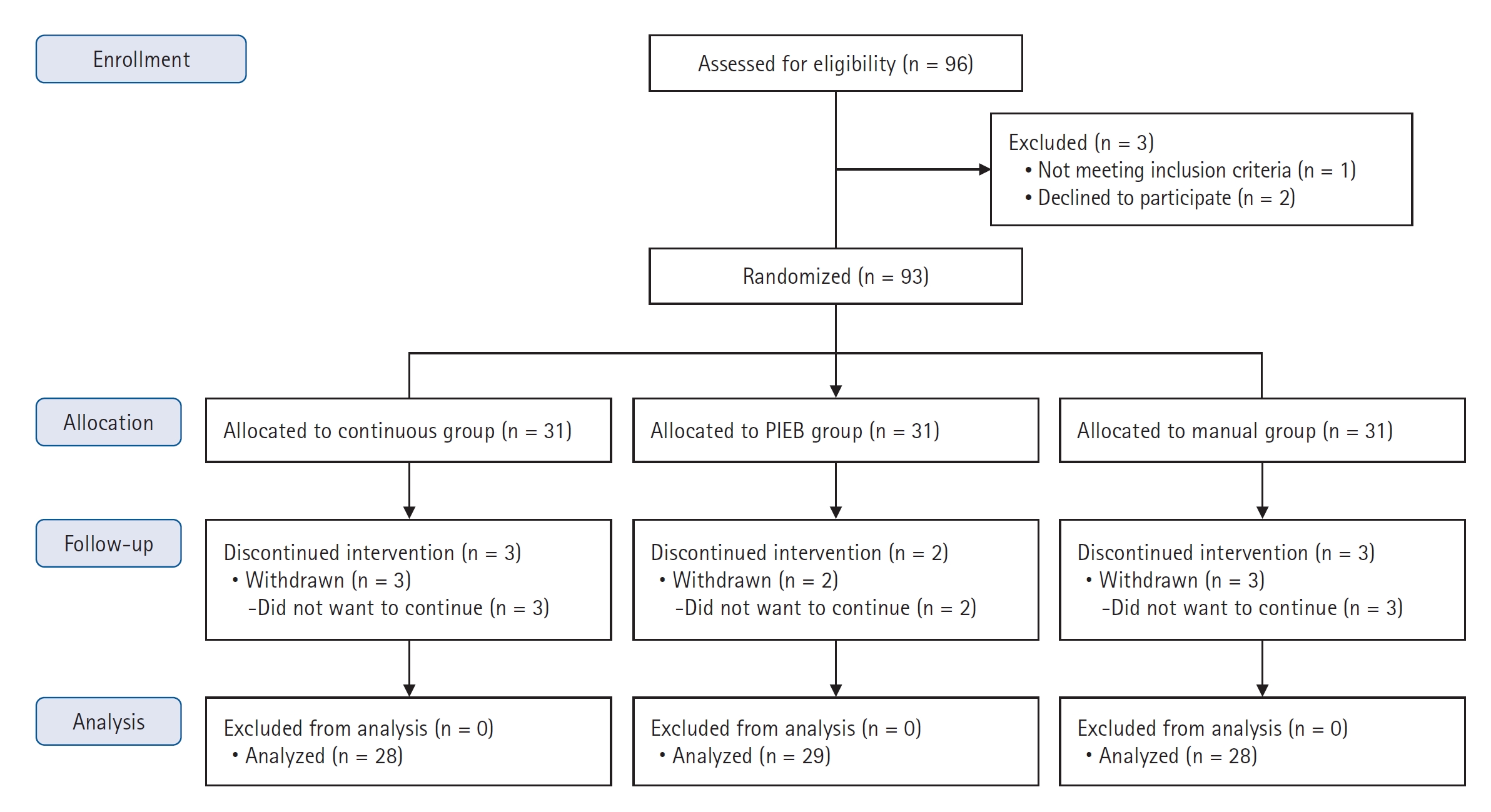
Study protocol
A combined spinal epidural analgesia (CSE) procedure for labor analgesia was performed by residents under the supervision of an experienced obstetrical anesthesiologist. Prior to the procedure, the patient’s intravenous route was secured, and standard monitoring was performed, including blood pressure, heart rate, pulse oximetry, respiration rate, fetal heart rate, and uterine contractions. At the L3–4 interspace with the patient in the lateral decubitus position, a lumbar puncture was performed using a 25-gauge Whitacre needle (BD® Whitacre spinal needle, 25 G × 3.50IN TW, BD). After confirming the free flow of cerebrospinal fluid, intrathecal agents (0.2% ropivacaine 3 mg with fentanyl 20 μg) were administered to relieve labor pain immediately. The epidural space was then located using a 17-gauge Tuohy needle at the L3–4 or L4–5 level with the loss of resistance to the air technique. An epidural catheter (FlexTip Plus® Epidural Catheterization Set, 19 G, Arrow Electronics) was inserted 5–6 cm into the epidural space, confirmed by negative aspiration of blood and cerebrospinal fluid and flushed with 4 mg of 0.2% ropivacaine. All the procedures were performed using aseptic techniques.
According to the assigned group, an ambulatory infusion pump (Accumate® 1200, Wooyoung Meditech Co., Ltd.), comprising 60 ml of 0.2% ropivacaine, fentanyl 180 μg, and 40 ml of 0.9% saline, was used. Prior to the start of this trial, an ambulatory infusion pump device was tested using an infusion device analyzer (IDA-4 Plus Multi-Channel Infusion Device Analyzer, Fluke® Biomedical) to confirm its applicability. Patient-controlled epidural analgesia (PCEA) was prepared by a nurse who was not involved in the trial.
The details of drug delivery protocol according to the assigned group were as follows. In the continuous group, PCEA + basal continuous epidural infusion 10 ml/h was started 30 min after the labor analgesia procedure. When the bolus button was pressed by the patient, 5 ml of the local anesthetic was injected. Continuous basal epidural infusion was continued, regardless of the bolus dose.
In the PIEB group, PCEA + PIEB 10 ml during one hour (240 ml/h for bolus infusion of 10 ml) and infusion were started 60 min after the labor analgesia procedure. When the bolus button was pressed by the patient, 5 ml of local anesthetic was injected and a PIEB was injected after 15 min.
In the manual group, PCEA + provider-administered intermittent epidural boluses of 10 ml during one hour (1,200 ml/h for bolus injection of 10 ml) and manual injection was started 60 min after the labor analgesia procedure. In the manual group, an experienced anesthesiologist injected 10 ml of ropivacaine with a fentanyl mixture at a constant rate for 30 s through an epidural catheter. When the bolus button was pressed by the patient, 5 ml of the local anesthetic was injected. A provider-administered epidural bolus was injected at set intervals regardless of the bolus dose.
Labor pain was measured using an 11-point numerical rating scale (NRS: 0 = no pain and 10 = the worst pain imaginable). The participants were informed that the PCEA bolus could be used for labor analgesia. Breakthrough pain was defined as pain requiring a bolus infusion of PCEA while receiving epidural anesthetics according to the assigned group. When breakthrough pain with an NRS score ≥ 4 occurred during PCEA infusion, rescue medications were injected as follows: 0.2% ropivacaine 14 mg was administered into the epidural space. If the pain did not subside, 50 mg of 1% lidocaine was administered. The delivery method was switched from vaginal delivery to Cesarean section in cases where failure to progress in labor occurred even after more than 4 h of labor or when the mother requested it.
Patients’ age, height, weight, body mass index, gestational age, cervical dilatation at the time of labor analgesia, total labor duration, any adverse effects associated with labor analgesia (e.g., nausea, vomiting, numbness, paraplegia, postdural puncture headache, and local anesthetic systemic toxicity), duration of second stage, incidence and NRS score of breakthrough pain, use of oxytocin, preoperative blood pressure, heart rate, NRS score after the labor analgesia procedure, conversion rate to Cesarean section, and patient satisfaction using a Likert scale were also recorded.
Statistical analyses
A power calculation was based on a previous study that investigated the effect of PCEA plus automated mandatory boluses (PIEB) for reducing the hourly consumption of local anesthetics during labor (mean ± standard deviation [SD]: control group, 7.5 ± 2.0 ml vs. PIEB group, 6.5 ± 3.4 ml) [
8]. We hypothesized that the difference in hourly local anesthetic consumption among the three groups would be clinically significant at a minimum of 1 ml. Thus, we calculated that 28 patients per group would provide a power of 80% at a significance level of 5%, under the assumption that the difference in local anesthetic consumption among the three groups was clinically significant. Considering a dropout rate of 10%, a minimum of 31 patients in each group (n = 93) were required to participate in the study.
Continuous variables are expressed as mean ± SD, median (Q1, Q3), or median (min, max) while normality was assessed using the Shapiro–Wilk test. For motor blockade and NRS score 4 hours after labor analgesia induction, the min and max values were additionally described. Categorical variables are expressed as numbers (percentages). A one-way analysis of variance or Kruskal–Wallis test was used as appropriate to determine the differences in continuous variables among the study groups, including hourly consumption of epidural analgesics, time interval to the first breakthrough pain, NRS score for breakthrough pain after labor analgesia, degree of sensory and motor nerve blockade, NRS score at 4 h after the labor analgesia procedure, and obstetric and neonatal outcomes. In case of statistical differences among the three groups, multiple comparisons were performed using Bonferroni correction. Categorical variables, including the incidence of breakthrough pain and mode of delivery, were analyzed using Pearson’s chi-square test. The partitioned chi-square test was used for multiple pairwise comparisons. Bonferroni correction was used to adjust P values for multiple comparisons. Statistical analyses were performed using SPSS® version 25 (IBM® Inc.), and P < 0.05 was considered significant.