Dexpanthenol protects against lipopolysaccharide-induced acute kidney injury by restoring aquaporin-2 levels via regulation of the silent information regulator 1 signaling pathway
Article information
Abstract
Background
Acute kidney injury (AKI) is a serious pathology that causes dysfunction in concentrating urine due to kidney damage, resulting in blood pressure dysregulation and increased levels of toxic metabolites. Dexpanthenol (DEX), a pantothenic acid analog, exhibits anti-inflammatory and anti-apoptotic properties in various tissues. This study investigated the protective effects of DEX against systemic inflammation-induced AKI.
Methods
Thirty-two female rats were randomly assigned to the control, lipopolysaccharide (LPS), LPS+DEX, and DEX groups. LPS (5 mg/kg, single dose on the third day, 6 h before sacrifice) and DEX (500 mg/kg/day for 3 days) were administered intraperitoneally. After sacrifice, blood samples and kidney tissues were collected. Hematoxylin and eosin, caspase-3 (Cas-3), and tumor necrosis factor alpha (TNF-α) staining were performed on the kidney tissues. The total oxidant status (TOS) and total antioxidant status were measured using spectrophotometric methods. Aquaporin-2 (AQP-2), silent information regulator 1 (SIRT1), and interleukin-6 (IL-6) were detected using quantitative reverse transcription-polymerase chain reaction analysis.
Results
Histopathological analysis revealed that DEX treatment ameliorated histopathological changes. In the LPS group, an increase in the blood urea nitrogen, creatinine, urea, IL-6, Cas-3, TNF-α, and TOS levels and oxidative stress index was observed compared with the control group, whereas AQP-2 and SIRT1 levels decreased. DEX treatment reversed these effects.
Conclusions
DEX was found to effectively prevent inflammation, oxidative stress, and apoptosis in the kidneys via the SIRT1 signaling pathway. These protective properties suggest DEX’s potential as a therapeutic agent for the treatment of kidney pathologies.
Introduction
Lipopolysaccharide (LPS) is an oligosaccharide extracted from the cell wall of Gram-negative bacteria that initiates the production and release of cytokines and subsequent inflammatory processes [1]. LPS interacts with toll-like receptor-4 (TLR-4) located on the surface of the host cell, inducing the secretion of various proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) [2]. This leads to the development of an inflammatory mechanism that could become systemic depending on the severity and/or duration. The kidneys, which are important elimination organs that receive the majority of blood, are also affected by this condition [3].
Acute kidney injury (AKI) is a clinical condition characterized by the sudden loss of kidney function, leading to an inability to eliminate urea and other nitrogenous waste products from the body along with alterations in extracellular fluid volume and electrolyte composition [4]. AKI can be caused by systemic inflammation, which is responsible for pathological changes, such as hyperemia, hemorrhage, and degeneration of tubular epithelial cells in the renal parenchyma [5]. This condition results in a decrease in glomerular filtration capacity and an increase in urea, blood urea nitrogen (BUN), and creatinine (Cr) levels due to impaired kidney function caused by inflammation [6].
Various water channels play a role in the development of clinical findings in renal pathology. Aquaporin-2 (AQP-2), a type of water channel found in the kidneys, plays a critical role in maintaining body water homeostasis [7]. The deficiency of AQP-2 is observed in various kidney pathologies such as AKI, diabetic nephropathy, nephrogenic diabetes insipidus, polycystic kidney disease, and renal cell carcinoma [8]. The existing literature indicates that restoration of AQP-2 levels through silent information regulator 1 (SIRT1) can alleviate kidney damage caused by inflammation [9].
SIRT1 is a member of the NAD+-dependent class III deacetylase family. The regulation of SIRT1 expression is involved in various biological processes such as inflammation, oxidative stress, and apoptosis [10]. Thus, decreased SIRT1 levels caused by inflammation may trigger oxidative stress and apoptosis. Tissue damage results in the formation of reactive oxygen species (ROS) that attempt to react and stabilize [11]. In pathological conditions where the antioxidant response, expressed as the total antioxidant status (TAS), is inadequate, the total oxidant status (TOS) increases, leading to oxidative stress [12]. A reduction in SIRT1 activation results in oxidative stress. Consequently, apoptosis cannot be prevented, leading to controlled cell death. Apoptosis occurs when a cell loses its capacity to prevent the spread of cellular dysfunction and damage. The caspase family of proteins is involved in apoptosis and regulated by SIRT1 [13]. This signaling pathway operates differently according to the intrinsic or extrinsic pathway of apoptosis, ultimately leading to cell death with caspase-3 (Cas-3) as the crucial mediator [14].
Dexpanthenol (DEX) is a biologically active alcohol analog of pantothenic acid that is converted into pantothenic acid in tissues. Studies have shown that DEX has anti-inflammatory, antioxidant, and anti-apoptotic effects in various tissues [15,16]. The potential relationship between DEX and SIRT1, which plays a role in the development of all pathologies associated with kidney damage, raises intriguing questions. This study aimed to demonstrate the relationship between DEX and SIRT1 and determine the protective effects of DEX on the kidneys via the SIRT1/AQP-2 signaling pathway.
Materials and Methods
Ethical approval
All experiments conducted in this study were performed in accordance with the National Institutes of Health guidelines for animal research and approved by the Suleyman Demirel University Isparta Committee on Animal Research (Approval No. 2022-06/72). This study was supported by the Scientific Research Fund of Suleyman Demirel University, under Project Number TSG-2020-8134.
Study animals and design
The sample size was calculated using the G Power 3.1.9.7 program (Heinrich-Heine-Universität Düsseldorf, Germany) with the following parameters: effect size = 0.4, alpha = 0.05, expected power (1-beta) = 0.80, and number of groups = 8.
Thirty-two adult female Wistar albino rats weighing 300–350 g were placed in a room under controlled temperature (21–22°C) and humidity (60% ± 5%) conditions, with a 12:12 h light/dark cycle. All the rats were fed a standard commercial chow diet (Korkuteli Yem, Turkey).
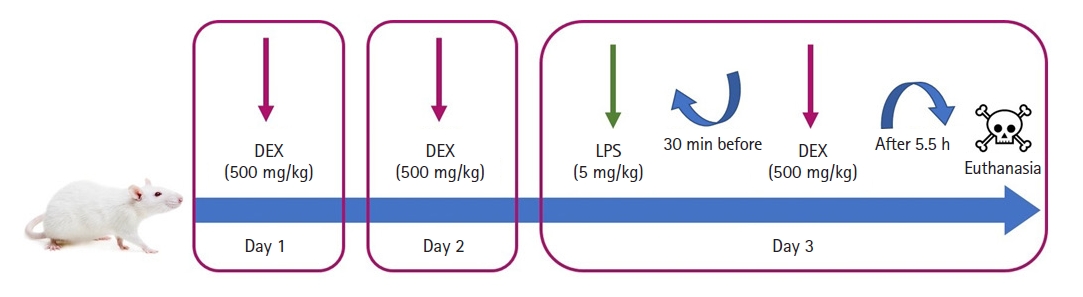
The rats in the control group (n = 8) received 1 ml of saline intraperitoneally (IP) once a day for three days in the left inguinal region and a single dose of 1 ml saline IP in the right inguinal region 30 min before the last dose of saline on the third day.
The rats in the LPS group (n = 8) received 1 ml of saline IP once a day for three days in the left inguinal region and a single dose of 5 mg/kg (0.5–1 ml) LPS (048K4126, Sigma Aldrich, USA) IP in the right inguinal region 30 min before the last dose of saline on the third day [17].
The rats in the LPS+DEX group (n = 8) received 1 ml of 500 mg/kg DEX (Bepanthen, Bayer, Turkey) IP once a day for three days in the left inguinal region and a single dose of 5 mg/kg (0.5–1 ml) LPS IP in the right inguinal region 30 min before the last dose of DEX on the third day [18].
The rats in the DEX group (n = 8) received 1 ml of 500 mg/kg DEX IP once a day for three days in the left inguinal region and a single dose of 1 ml saline IP in the right inguinal region 30 min before the last dose of DEX on the third day.
Six hours after LPS (or saline for control and DEX groups) administration, all rats received 80–100 mg/kg ketamine (Ketalar, Pfizer, Turkey) and 8–10 mg/kg xylazine (XylazinBio 2%, Bioveta PLC, Czech Republic) to induce anesthesia. After anesthesia, the rats were sacrificed and blood samples were taken from the inferior vena cava through an abdominal incision. Half of the kidney specimens were stored at –20°C for biochemical analysis and –80°C for quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis, while the remaining tissues were fixed with 10% buffered formalin for histopathological and immunohistochemical analysis. Experimental design has been visualized in Fig. 1.
Histopathological examination
Kidney specimens were fixed in a 10% buffered formalin solution. Samples were collected for routine pathological processing using an automatic tissue processor (Leica ASP300S, Leica Microsystems, Germany) and embedded in paraffin wax. Using a rotary microtome (Leica RM2155, Leica Microsystems), 5-µm thick sections were obtained from the paraffin blocks. The sections were then stained with hematoxylin and eosin (H&E), mounted on a coverslip, and examined under a light microscope.
Immunohistochemical examination
For immunohistochemical examination, two series of sections taken from all blocks of the kidneys were drawn on poly-L-lysine coated slides and stained immunohistochemically for Cas-3 (anti-caspase-3 antibody (E-8): sc-7272) and TNF-α (anti-TNFα antibody (52B83): sc-52746, 1/100 dilution) (Santa Cruz Biotechnology, Inc., USA) expression using the streptavidin biotin technique according to the manufacturer’s instructions. The sections were incubated with primary antibodies for 60 min and immunohistochemistry was performed using biotinylated secondary antibodies and streptavidin-alkaline phosphatase conjugate. EXPOSE mouse- and rabbit-specific HRP/DAB detection IHC kits (ab80436) (Abcam, UK) were used as secondary antibodies. Diaminobenzidine (DAB) was used as the chromogen. For the negative controls, an antigen dilution solution was used instead of the primary antibody. All examinations were performed on blinded samples by a specialized pathologist from another university.
For immunohistochemical analysis, the sections were investigated separately for each antibody. To evaluate the severity of the immunohistochemical reaction of cells with markers, semi-quantitative analyses were performed using a grading score ranging from (0) to (3) as follows: (0) negative staining, (1) focal weak staining, (2) diffuse weak staining, and (3) diffuse strong staining. For evaluation, 10 different areas of each sections examined under 40x objective magnification. Morphometric analyses and microphotography were performed using the Database Manual cellSens Life Science Imaging Software (Olympus Opitical Co., Japan). The results were saved and statistically analyzed.
Measurement of blood parameters
The blood samples of all the individual rats (n=32) were transferred into gel-containing tubes, and the sera were separated by centrifugation at 3,000 rpm for 10 min and aliquoted into three portions per serum. Samples were stored at –80°C until they were analyzed. Urea and Cr levels were measured spectrophotometrically with a Beckman Coulter AU 5800 autoanalyzer (Beckman Coulter, USA) using a kit compatible with the instrument (Beckman Coulter Urea/Urea Nitrogen assay kit, Beckman Coulter Creatinine assay kit) and BUN levels were calculated with the formula BUN = Urea/2.14.
Measurement of oxidative stress parameters
Kidney tissue samples were homogenized using the Ultra Turrax Janke & Kunkel T-25 homogenizer (IKA®-Werke GmbH & Co. KG, Germany) for oxidant-antioxidant analysis. The TAS and TOS were measured spectrophotometrically (AU 5800 autoanalyzer, Beckman Coulter) using commercial kits (Rel Assay Diagnostics, Turkey), and the oxidative stress index (OSI) was calculated using the following formula: OSI = TOS/TAS/10 [19]. For TAS analysis, antioxidants in the sample reduce dark blue-green colored 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals to their colorless ABTS form. The change in the absorbance at 660 nm was associated with the TAS level of the sample. This method determines the antioxidative effect of a sample against potent free radical reactions initiated by the production of hydroxyl radicals. The results are expressed as µmol Trolox Eqv/L [20].
For TOS analysis, oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to the ferric ion. Oxidation reactions are enhanced by glycerol molecules, which are abundant in the reaction medium. The ferric ions form a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, has been found to be related to the total amount of oxidant molecules already present in the sample. The assay was calibrated with hydrogen peroxide, and the results were expressed in terms of micromolar hydrogen peroxide equivalents per liter (µmol H2O2 Eqv/L) [21].
Quantitative reverse transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues using TRIzolTM Reagent (#15596026, Thermo Fisher Scientific, Life Sciences Solutions, USA) according to the manufacturer’s instructions. The quality of the RNA was determined using a nanospectrophotometer (#732-2533 VWR® mySPEC, Micro-Volume Spectrophotometers, VWR International GmbH, Austria). RNA (1 μg) was transcribed using the iScript cDNA Synthesis Kit with oligo-dT primers (Bio-Rad Laboratories, USA). The reaction mixture was incubated for 20 min at 46°C, 5 min at 25°C, and 1 min at 95°C. Real-time PCR amplification was performed using the SYBR Green Master Mix (Bio-Rad Laboratories) according to the manufacturer’s instructions using a CFX96 instrument (Bio-Rad Laboratories). Specific primers were designed for the amplification of SIRT1, AQP-2, IL-6 (primer sequences provided upon request) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the cDNA samples for each PCR were analyzed in triplicate. GAPDH expression was used for normalization. Gene expression was relatively quantified using the comparative ΔΔCt method.
Statistical analysis
Statistical analyses between groups were performed using the SPSS 18.00 package program (SPSS Inc., USA) and the one-way analysis of variance (ANOVA) post hoc LSD test was used for the qRT-PCR, biochemical, histopathological, and immunohistochemical analyses. The Kruskal–Wallis test was used to identify any notable differences in the immunohistochemical analyses among the groups. Statistical significance was set at P < 0.05.
Results
Histopathological results
Histopathological examination revealed marked hyperemia, slight to moderate hemorrhage, and degeneration of tubular epithelial cells in kidney sections of the LPS group. In addition to inflammatory cell infiltration, DEX treatment decreased the pathological findings in the LPS+DEX group. Normal tissue histology was observed in the control and DEX groups (Fig. 2).
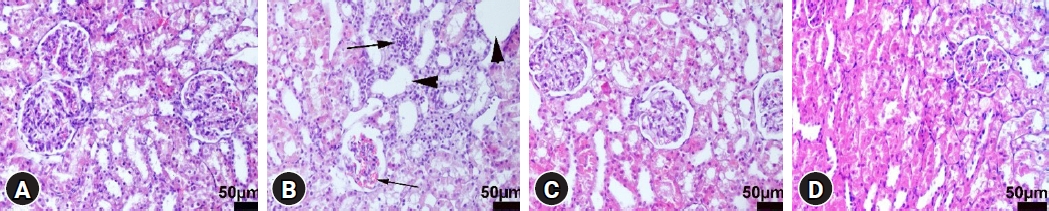
Microscopical appearance of the kidneys among the groups. (A) Normal tissue histoarchitecture in the control group, rat number: 2. (B) Marked hyperemia in the glomerulus (thin arrow), inflammatory cell infiltrations (thick arrow), and cystic dilatations in the tubules (arrow heads) in the LPS group, rat number: 1. (C) Decreased pathological findings in the LPS+DEX group, rat number: 4. (D) Normal kidney histology in the DEX group, rat number: 6. HE, scale bars = 50 µm. LPS: lipopolysaccharide, DEX: dexpanthenol.
Immunohistochemical findings
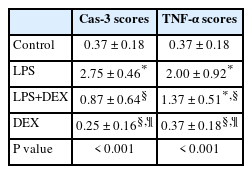
Immunohistochemical examination showed increased Cas-3 and TNF-α expression in tubular cells in the LPS group. DEX treatment ameliorated these changes in the LPS+DEX group. Very low or negative expression was observed in the control and DEX groups (Figs. 3 and 4). The results of the statistical analyses are presented in Table 1.
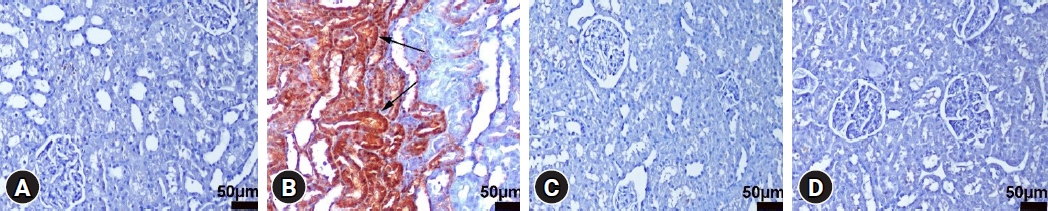
Cas-3 immunohistochemical findings of the kidneys among the groups. (A) Negative expression in the control group, rat number: 3. (B) Marked increase in the expression (arrows) of tubular epithelial cells in the LPS group, rat number: 7. (C) Decreased expression in the LPS+DEX group, rat number: 3. (D) Negative expression in the DEX group, rat number: 2. Streptavidin biotin peroxidase method, scale bars = 50 µm. LPS: lipopolysaccharide, DEX: dexpanthenol.
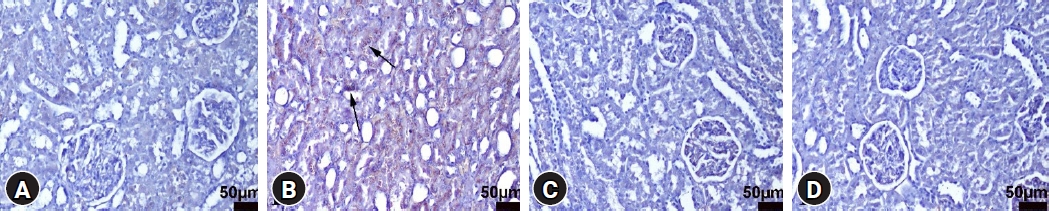
TNF-α immunohistochemistry findings of the kidneys among the groups. (A) Negative expression in the control group, rat number: 1. (B) Increased expression (arrows) of the tubular epithelial cells in the LPS group, rat number: 2. (C) Decreased expression in the LPS+DEX group, rat number: 7. (D) Negative expression in the DEX group, rat number: 7. Streptavidin biotin peroxidase method, scale bars = 50 µm. LPS: lipopolysaccharide, DEX: dexpanthenol.
Biochemical results
Urea, Cr, and BUN levels were measured biochemically to evaluate kidney function. All markers were significantly increased in the LPS group compared to the control group (P < 0.001, P < 0.01, and P < 0.001, respectively). DEX treatment significantly reversed these changes (P < 0.001, P < 0.01, and P < 0.001, respectively). All kidney function markers were found to be significantly lower in the DEX group than in the LPS group (P < 0.001, P < 0.01, and P < 0.001, respectively) (Fig. 5).

BUN, Cr, and Urea levels. Values are presented as mean ± SD. LPS: lipopolysaccharide, DEX: dexpanthenol, BUN: blood urea nitrogen, Cr: creatinine. *P < 0.01, †P < 0.001.
To indicate oxidative stress in the kidney tissues, TOS and TAS levels were measured, and the OSI was calculated. The increase in the LPS group was significant compared to the control group for TOS levels and the OSI (P < 0.001 for both). DEX treatment significantly reversed these effects (P < 0.01 and P < 0.001, respectively) (Fig. 6).

Oxidative stress markers in kidney tissue. Values are presented as mean ± SD. LPS: lipopolysaccharide, DEX: dexpanthenol, TOS: total oxidant status, TAS: total antioxidant status, OSI: oxidative stress index. *P < 0.01, †P < 0.001.
SIRT1, AQP-2, and IL-6 levels were evaluated using qRT-PCR. In the LPS group, SIRT1 and AQP-2 levels significantly decreased, whereas IL-6 levels significantly increased (P < 0.001, P < 0.01, and P < 0.01, respectively). Although DEX treatment reversed these effects, only the increase in AQP-2 was significant (P < 0.01). IL-6 levels in the LPS+DEX group were significantly higher compared to the control group (P < 0.05). In the DEX group, SIRT1 and AQP-2 levels were the highest, while IL-6 levels were the lowest (Fig. 7).
Discussion
The interplay between systemic inflammation and endothelial and tubular cell injury is crucial for AKI pathogenesis [22]. The development of tubular dysfunction due to microvascular injury leads to disruption of renal excretion, thereby affecting the blood profile [23]. This can also result in the dysfunction of AQP channels as a consequence of tubular damage. Systemic inflammation is a major cause of AKI and can trigger this pathological process [24]. This study aimed to demonstrate the therapeutic effects of DEX against renal damage induced by systemic inflammation.
The recognition of LPS by TLR-4 in systemic circulation triggers an immune response and leads to the release of inflammatory cytokines [25]. Because the kidneys receive the most blood per unit of tissue, they are inevitably affected by this systemic event [26]. In our study, we observed that the levels of the most important inflammatory cytokines, TNF-α and IL-6, were elevated in the LPS group, and the potential treatment agent, DEX, reversed these effects owing to its anti-inflammatory activity. These findings are consistent with those of Bilgic et al. [27], who reported a significant decrease in TNF-α and IL-6 levels following DEX treatment in a rat model of drug toxicity.
Although the molecular relationship between systemic inflammation and AKI is not fully understood, several mechanisms have been proposed. Tubular degeneration may significantly affect active and passive transport by impairing tubular integrity and water channel function, resulting in problems with the excretion of urea and similar metabolites from the kidneys. In this study, an increase in urea, BUN, and Cr levels was observed in the AKI model induced by inflammation, and an improvement was observed with DEX treatment.
Furthermore, previous studies have shown that decreased AQP-2 levels are associated with kidney disease and that restoring AQP-2 levels can have therapeutic effects [28]. Consistent with these findings, an increase in AQP-2 levels in the DEX group compared with the LPS group was found in the current study. An in vitro study demonstrated that this increase was mediated by SIRT1 and our results are consistent with these findings [29].
The association between inflammation and oxidative stress is well established, and microvascular damage may contribute to this process [30]. Microvascular damage can lead to the impairment of tubular function and tissue oxygenation, resulting in the generation of ROS that exacerbates inflammation and tissue damage [31]. The decrease in the OSI following DEX treatment in our study is thus consistent with previous study, which have reported that DEX decreases OSI in a model of amikacin-induced nephrotoxicity [18]. The imbalance in oxidative stress is dependent on an insufficient response of the SIRT1 signaling pathway, which plays a crucial role in regulating inflammation and apoptosis. The decrease in SIRT1 levels observed in the LPS group was associated with changes in inflammation, oxidative stress, and apoptosis markers, as evidenced by an increase in Cas-3 levels. Our findings suggest that DEX treatment restores SIRT1 levels and exerts a curative effect against AKI-induced inflammation, oxidative stress, and apoptosis.
In conclusion, the results of this study suggest that DEX treatment can prevent the formation of damage mechanisms in AKI induced by systemic inflammation triggered by LPS by restoring AQP-2 levels via the SIRT1 signaling pathway, thus protecting the kidney structure in rats. Therefore, DEX may be a promising candidate for treating AKI and other kidney diseases associated with reduced AQP-2 levels. However, the lack of significant changes in the group treated with DEX alone suggests the need for further studies assessing different durations and doses of DEX. Detailed molecular analyses are required to fully understand the mechanisms underlying the therapeutic effects of DEX on renal damage.
Notes
Funding
This work was supported by the Scientific Research Projects Coordination Unit of Suleyman Demirel University (Project number TSG-2020-8134).
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Eyyüp Sabri Özden (Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Halil Aşcı (Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Halil İbrahim Büyükbayram (Data curation; Formal analysis; Methodology; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Mehmet Abdulkadir Sevük (Data curation; Investigation; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing)
Orhan Berk İmeci (Data curation; Investigation; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing)
Hatice Kübra Doğan (Formal analysis; Methodology; Software; Writing – original draft; Writing – review & editing)
Özlem Özmen (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)