Total postoperative opioid dose is an independent risk factor for prolonged postoperative ileus after laparoscopic colorectal surgery: a case-control study
Article information
Abstract
Background
Prolonged postoperative ileus (PPOI) is a major complication of colorectal surgery. Increased opioid consumption has been proposed to increase the risk of PPOI. This study aimed to test the hypothesis that an increased total postoperative opioid dose (TPOD) is associated with the increased incidence of PPOI.
Methods
For this matched case-control study, patients who underwent elective laparoscopic colorectal procedures at the Peking University People’s Hospital between January 2018 and June 2020 were retrospectively reviewed. Patients with PPOI were assigned to the ileus group, while patients without PPOI (control group) were matched at a 1:1 ratio to the ileus group according to age, American Society of Anesthesiologists physical status score, and type of surgical procedure. The primary outcome was the TPOD between the ileus and control groups. The secondary outcome was risk factors of PPOI.
Results
A total of 267 participants were included in the final analysis. No differences in baseline or operative factors were found between the two groups. The TPOD, intravenous sufentanil dose on postoperative day 1 (POD1), and the use of patient-controlled analgesia with basal infusion were associated with PPOI (P < 0.05). Multivariate logistic regression analysis revealed that an increased TPOD was an independent risk factor for developing PPOI after laparoscopic colorectal procedures (Odd ratio: 1.67, 95% CI [1.03, 2.71], P = 0.04).
Conclusions
The TPOD is an independent risk factor for PPOI after laparoscopic colorectal surgery. We need to explore new strategies of postoperative analgesia to reduce the dosage of TPOD.
Introduction
Prolonged postoperative ileus (PPOI) is a major complication of colorectal surgery, with a reported prevalence of 10%–30%. PPOI leads to increased morbidity and duration of hospital stay (DoHS), thereby increasing medical costs [1–3]. Determining risk factors for PPOI is one of the key elements for the Enhanced Recovery after Surgery (ERAS) protocol.
Opioid receptors are present throughout the gastrointestinal tract. Activation of μ-receptors located in the enteric nervous system causes increased non-propulsive contractions and inhibition of water and electrolyte excretion. These actions lead to delayed gastrointestinal transit and hard, infrequent stools [4,5]. An increase in the perioperative opioid dose may be an independent risk factor for PPOI. Although opioids are widely used to attenuate intraoperative stress and represent the cornerstone of pain treatment, anesthesiologists are striving to reduce perioperative opioid consumption to decrease opioid-related gastrointestinal side effects. Minimizing opioid use has been suggested to reduce PPOI risk [6].
Several strategies have been proposed to reduce opioid consumption and improve recovery of bowel function. First, multimodal pain management is recommended whenever possible. Second, a neuraxial or peripheral nerve block [7–9], subcutaneous infiltration of local anesthetics, and use of nonsteroidal anti-inflammatory drugs or acetaminophen have been shown to be effective [10–12]. Patient-controlled analgesia (PCA) without a basal infusion may also be an effective strategy [13].
This study aimed to test the hypothesis that an increased total postoperative opioid dose (TPOD) is associated with increased incidence of PPOI.
Materials and Methods
The protocol for this single-center, matched case-control study was approved by the Ethics Review Board of Peking University People’s Hospital in Beijing, China (2021PHB144-001). The requirement for written informed consent was waived due to the retrospective design of the study. The study was conducted according to the STROBE criteria and registered at www.clinicaltrials.gov (NCT05262569).
All consecutive patients who underwent an elective laparoscopic colorectal procedure at the Department of Gastroenterologic Surgery at Peking University People’s Hospital in Beijing, China between January 1, 2018 and June 30, 2020 were included. Patients who met any of the following criteria were excluded: (i) long-term opioid use, (ii) conversion from a laparoscopic to an open procedure, (iii) admission to the intensive care unit (ICU), (iv) opioids other than sufentanil included in the PCA regimen, (v) PCA use for < 48 h, and (vi) missing data on post-surgery opioid consumption.
Patients with PPOI were included in the ileus group. In accordance with the PPOI definition proposed by Vather et al. [14], patients who met ≥ 2 of the following 5 criteria on postoperative day (POD) 4 or later had PPOI: (i) nausea and vomiting over the preceding 12 h, (ii) inability to tolerate a solid or semi-solid diet over the preceding two mealtimes, (iii) abdominal distension, (iv) failure to pass gas or stool for a 24 h period, and (v) radiological evidence of an ileus in the preceding 24 h, without postoperative pain management, returned to operation room before discharge.
The control and ileus groups were matched at a 1:1 ratio for the following: age range (± 5 years), American Society of Anesthesiologists (ASA) physical status score, and type of surgical procedure (colectomy, rectal resection, whole-colon resection, or others).
All patients underwent a standard anesthesia protocol for laparoscopic colorectal procedures at the study center. The opioid administered intraoperatively was sufentanil 0.3–0.5 µg/kg during induction and 0.1 µg/kg before skin incision and/or upon skin closure. Remifentanil 0.1–0.2 µg/kg/min continuous infusion was administered for maintenance of anesthesia. Postoperative pain was managed with patient-controlled intravenous analgesia using sufentanil with or without basal infusion, at the discretion of the anesthesiologist.
Data on baseline factors, operative factors, and analgesia-related risk factors were obtained from electronic medical records. Multiple potential risk factors for PPOI were considered based on a review of the literature.
Baseline factors included age, sex, body mass index, presence of major comorbidities (cardiovascular diseases, cerebral diseases, pulmonary diseases, or diabetes mellitus), ASA physical status scores, and history of abdominal surgery. Operative factors included the type and duration of the surgical procedure, estimated blood loss, total input, time to tolerance of an oral diet, and postoperative DoHS. Analgesia-related protective or risk factors included a transversus abdominis plane (TAP) block, intraoperative opioid consumption (converted into equivalent doses of morphine in mg/kg according to morphine 1 mg = oxycodone 0.5 mg = fentanyl 10 μg = sufentanil 1 μg = remifentanil 10 µg) [15], intravenous opioid dose (sufentanil, μg/kg) on POD1 through PCA, TPOD (sufentanil, μg/kg) through PCA, and basal infusion on PCA.
The primary outcome was the TPOD (sufentanil, μg/kg) between the ileus and control groups. Secondary outcomes included administration of a TAP block, intravenous opioid dose (sufentanil, μg/kg) on POD1, and PCA with basal infusion.
Parametricity was determined using the Shapiro-Wilk test, with normally distributed data expressed as the mean ± standard deviation (SD) and non-parametric data as the median ± interquartile range. Categorical variables are presented as numbers and percentages. Between-group differences were evaluated using the independent t-test or Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Univariate and multivariate analyses were performed using conditional logistic regression to determine potential risk factors for PPOI. Variables that were significant (P < 0.05) in the univariate analysis were included in the multivariate analysis to determine the independent risk factors for PPOI. The results are presented as odds ratios (ORs) with 95% CIs. Statistical significance was set at P < 0.05 for all outcomes. The power of this study was calculated using http://sample-size.net, with the TPOD (sufentanil, μg/kg) as the primary outcome. All the statistical analyses were performed using SPSS version 23 (IBM Corp.).
Results
Patient population and characteristics
A total of 596 consecutive cases were reviewed in the initial study, 267 of which were included after applying the exclusion criteria. Of these 267 patients, 15.0% (40/267) met the definition of PPOI according to the study protocol. Thirty-eight patients and controls were matched for age (± 5 years), ASA physical status scores, and type of surgical procedure (Fig. 1).
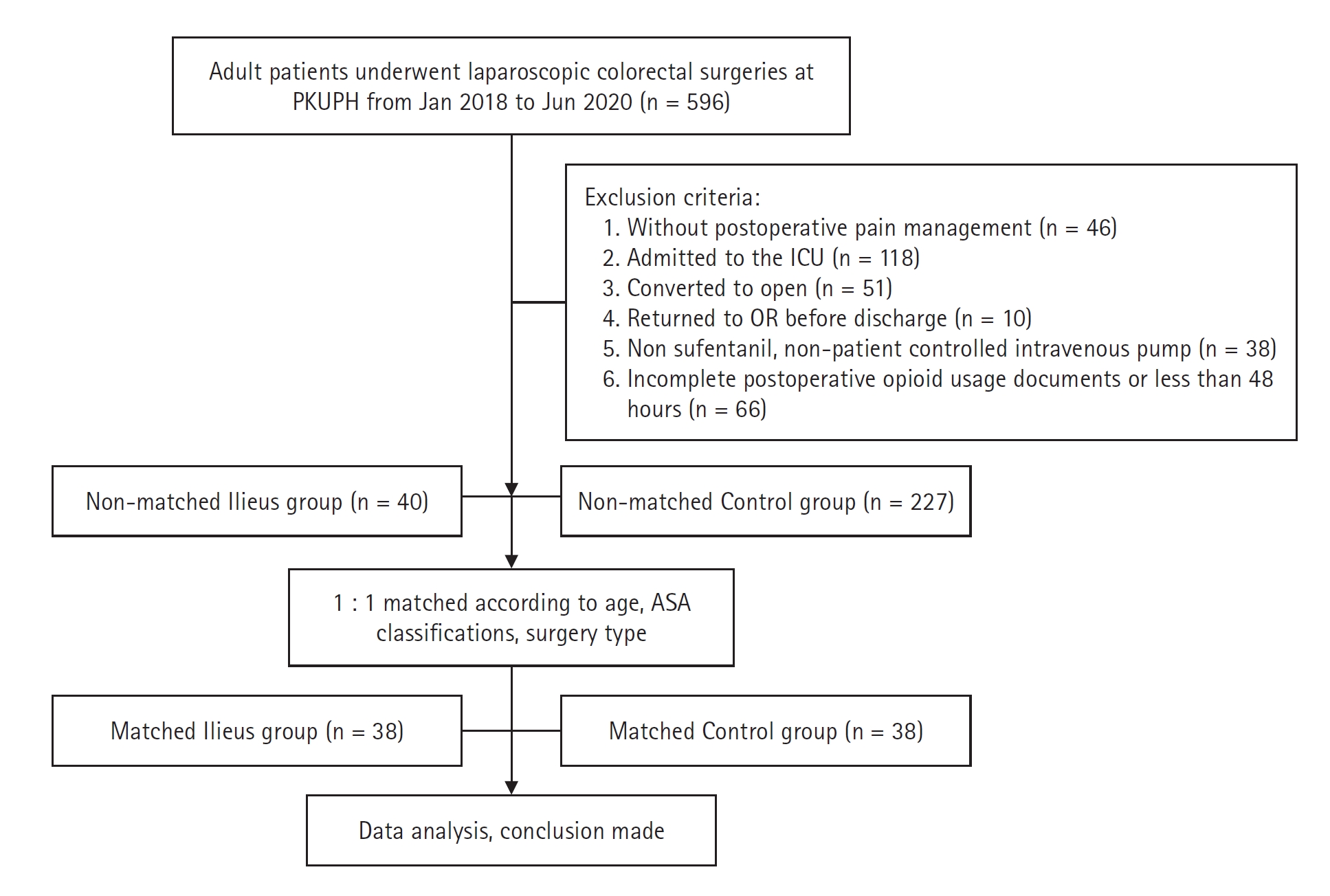
Flowchart of patient allocation. PUPH: Peking University People’s Hospital, ICU: Intensive care unit, OR: operating room, ASA: American Society of Anesthesiologists.
No significant differences in patient characteristics were found between the ileus and control groups (Table 1). The use of a TAP block was significantly lower in the ileus group than in the control group (6 vs. 14, P = 0.038). Patients used more sufentanil after surgery (2.3 ± 1.0 vs. 0.7 ± 1.0 μg/kg, P < 0.001) and the sufentanil dose was significantly higher on POD1 (0.9 ± 0.4 vs. 0.4 ± 0.5 μg/kg, P < 0.001) in the ileus group compared to the control group. Additionally, more patients in the ileus group received a PCA pump with a basal infusion (31 vs. 18, P = 0.002). The time to tolerance of oral intake (12 [9, 15] vs. 6 [5.75, 7] days) and postoperative DoHS (14 [11, 19.25] vs. 8 [7, 9] days) were significantly longer in the ileus group (P < 0.001).
The results of the univariate analysis are presented in Table 2. The TPOD (OR: 1.49, 95% CI [1.21, 1.85], P < 0.001), sufentanil dose on POD1 (OR: 2.31, 95% CI [1.37, 3.87], P = 0.002), and use of PCA with basal infusion (OR: 2.44, 95% CI [1.08, 5.54], P = 0.003) were associated with PPOI. Multivariate logistic regression analysis revealed that an increased TPOD was an independent risk factor for developing a PPOI after a laparoscopic colorectal procedure (OR: 1.67, 95% CI [1.03, 2.71], P = 0.04). Each 1 μg/kg increase in the sufentanil dose was associated with a 1.67-fold increase in the risk of PPOI.
Discussion
The current study confirmed the hypothesis that an increased TPOD is an independent risk factor for PPOI. The TPOD was significantly higher in the ileus group, which is consistent with a study conducted by Artinyan et al. [16], who demonstrated that the TPOD is an independent predictor of PPOI. In that study, the mean TPOD of the study population was 2.36 mg/kg of morphine, which was consistent with the value in our ileus group (mean TPOD of sufentanil was 2.3 μg/kg, which is equivalent to 2.3 mg/kg of morphine).
In our study, we controlled for other potential confounders for PPOI by matching the age [17], ASA score [18], and type of surgical procedure [19] between the groups. We excluded the patients whose procedure was converted to open surgery because open procedures involve more bowel handling [20] and patients admitted to the ICU since they receive different postoperative pain management strategies. This resulted in the inclusion of 38 balanced pairs for further analysis. As predicted, the time to tolerance of an oral diet and postoperative DoHS were significantly longer in the ileus group. These data encouraged us to explore strategies to minimize the postoperative opioid dose to decrease PPOI occurrence.
Two potential reasons for the association between an increased TPOD and PPOI in this study were postulated. First, fewer patients in the ileus group received a TAP block preoperatively (15.8% vs. 36.8%, P = 0.04), which resulted in significantly higher opioid consumption during the first 24 h in the ileus group (0.9 ± 0.4 vs. 0.4 ± 0.5 μg/kg, P < 0.001). Preoperative TAP blocks have been demonstrated to reduce opioid consumption on POD1 [21]; therefore, the ERAS protocol strongly recommends the use of the TAP block for minimally invasive surgery [22]. We demonstrated that the lower prevalence of TAP blocks in the ileus group was associated with increased opioid consumption on POD1. This may have contributed to the slow recovery of bowel function in the ileus group, resulting in a longer time to tolerate an oral diet and a longer DoHS. Second, more patients in the ileus group received PCA with a basal infusion (81.6% vs. 47.4%, P = 0.002). Zhen et al. [23] previously demonstrated that including a basal infusion on PCA with sufentanil was efficacious and safe [23]. However, whether sufentanil influenced patient recovery (including gastrointestinal function) was not investigated in that study [23]. Sufentanil has recently become the primary opioid used at Peking University People’s Hospital postoperatively. Anesthesiologists usually administer a basal infusion of sufentanil because of its intermediate half-life. However, an increasing number of anesthesiologists are attempting to reduce opioid use to comply with ERAS recommendations. In recent years, one strategy has been to provide PCA without a basal infusion, which resulted in a lower TPOD and PPOI prevalence in the current study.
The primary strength of our study is the matched case-control design to control for confounding factors, thereby focusing on analgesic-related risk factors. However, our study also has three main limitations. First, the protocol for perioperative analgesia according to the ERAS guidelines has changed since this study was conducted [6], which could affect the prevalence of PPOI. Second, patients who were admitted to the ICU postoperatively were excluded as they were deemed to have more comorbidities and to have undergone more complicated procedures. Thus, the results of this study may not be generalizable. Third, our sample size was small; however, using the total postoperative dose of sufentanil as the primary endpoint, the power of our study was calculated to be as high as 100%.
In conclusion, the TPOD was found to be an independent risk factor of PPOI after laparoscopic colorectal procedures. Therefore, we need to explore new strategies of postoperative analgesia to reduce the dosage of TPOD.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The authors have placed an embargo on the public availability of the data due to ongoing secondary analyses.
Author Contributions
Hui Ju (Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Validation; Writing – original draft; Writing – review & editing)
Kai Shen (Data curation; Resources; Supervision)
Jiaxin Li (Formal analysis; Writing – original draft)
Yi Feng (Conceptualization)


