Effects of dexmedetomidine on pulmonary function in patients receiving one-lung ventilation: a meta-analysis of randomized controlled trial
Article information
Abstract
Background
Mechanical ventilation, particularly one-lung ventilation (OLV), can cause pulmonary dysfunction. This meta-analysis assessed the effects of dexmedetomidine on the pulmonary function of patients receiving OLV.
Methods
The Embase, PubMed, MEDLINE, Cochrane Library, ClinicalTrials.gov, and Chinese Clinical Trial Registry databases were systematically searched. The primary outcome was oxygenation index (OI). Other outcomes including the incidence of postoperative complications were assessed.
Results
Fourteen randomized controlled trials involving 845 patients were included in this meta-analysis. Dexmedetomidine improved the OI at 30 (mean difference [MD]: 40.49, 95% CI [10.21, 70.78]), 60 (MD: 60.86, 95% CI [35.81, 85.92]), and 90 min (MD: 55, 95% CI [34.89, 75.11]) after OLV and after surgery (MD: 28.98, 95% CI [17.94, 40.0]) and improved lung compliance 90 min after OLV (MD: 3.62, 95% CI [1.7, 5.53]). Additionally, dexmedetomidine reduced the incidence of postoperative pulmonary complications (odds ratio: 0.44, 95% CI [0.24, 0.82]) and length of hospital stay (MD: −0.99, 95% CI [−1.25, −0.73]); decreased tumor necrosis factor-α, interleukin (IL)-6, IL-8, and malondialdehyde levels; and increased superoxide dismutase levels. However, only the results for the OI and IL-6 levels were confirmed by the sensitivity and trial sequential analyses.
Conclusions
Dexmedetomidine improves oxygenation in patients receiving OLV and may additionally decrease the incidence of postoperative pulmonary complications and shorten the length of hospital stay, which may be related to associated improvements in lung compliance, anti-inflammatory effects, and regulation of oxidative stress reactions. However, robust evidence is required to confirm these conclusions.
Introduction
Mechanical ventilation, particularly one-lung ventilation (OLV), significantly reduces lung compliance and ventilation, which leads to pulmonary dysfunction ranging from temporary minor hypoxia to severe fatal manifestations (e.g., acute respiratory distress syndrome), especially in patients with pulmonary diseases [1,2]. Pulmonary dysfunction impairs patient outcomes and substantially increases the burden on the healthcare system regardless of its severity [3]. However, no protective modalities with consistent efficacy and safety are available at present [4]. Therefore, anesthesiologists continue to explore strategies to protect lung function.
Dexmedetomidine is a selective Alpha-2 agonist with various clinical uses in anesthesiology and intensive care [1]. Some studies have reported that in addition to its sedative and cardiovascular effects, dexmedetomidine also serves a protective function in respiratory mechanics and oxygenation both in animals [5–7] and in operative patients receiving mechanical ventilation [1,8,9]. However, another study showed that dexmedetomidine did not confer any protective effects on the lungs [10]. Therefore, this meta-analysis aimed to assess the effects of dexmedetomidine on pulmonary function in patients receiving OLV and provide reliable evidence for its clinical application.
Materials and Methods
This meta-analysis was conducted in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11] and was registered with the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42022352468). All modifications to the PROSPERO-registered protocol are described below.
Search strategy
The Embase, PubMed, Medline, Cochrane Library, ClinicalTrials.gov, and Chinese Clinical Trial Registry databases were comprehensively searched from their inception to October 21, 2022, by two reviewers (L.Y. and Y.C.) independently according to the search strategy (Supplementary Material 1), without restrictions on language or publication date. The search terms included the following: dexmedetomidine, respiratory, lung, pulmonary, breathing, respiration, oxygenation, PaO2/FiO2, P/F ratio, mechanics, compliance, dynamic compliance, Cdyn, resistance, peak inspiratory pressure, Ppeak, airway peak pressure, plateau pressure, dead space, transpulmonary pressure, intrapulmonary shunt, and Qs/Qt. Boolean logical operators were used to connect search terms. The references of identified trials and systematic reviews were also manually searched for additional potentially relevant trials.
Eligibility criteria
The inclusion criteria were as follows: (1) operative patients receiving OLV; (2) randomized controlled trials (RCTs), irrespective of language; (3) studies comparing the effects of intravenous dexmedetomidine infusion with placebo or blank infusion; and (4) studies with complete data on one of the following outcomes: PaO2/FiO2 or oxygenation index (OI), lung compliance, airway resistance, peak inspiratory pressure (Ppeak), plateau pressure (Pplat), dead space, transpulmonary pressure, and intrapulmonary shunt or Qs/Qt. Publications without full texts available or with unextractable data were excluded.
Data extraction
Two reviewers (L.Y. and Y.C.) independently used a standard data extraction form to retrieve relevant data. Discrepancies were identified and resolved through discussion with a third reviewer (B.C.) when necessary. The extracted data included details on the following: first author, country, study design, sample size, publication date, patient age and sex, interventions, type of surgery, inclusion and exclusion criteria, and outcomes.
The primary outcomes were the OI at 30, 60, and 90 min after OLV and after surgery. Secondary outcomes were lung compliance, airway resistance, Ppeak, Pplat, dead space ventilation, transpulmonary pressure, Qs/Qt, serum inflammatory factors, oxidative stress indices, mean arterial pressure (MAP), and heart rate (HR) at 30, 60, and 90 min after OLV and after surgery; postoperative pulmonary complications; and length of hospital stay.
Assessment of methodological quality
Two reviewers (L.Y. and Y.C.) independently assessed the quality of the RCTs based on the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions. A “risk of bias” table, which included details on the methods used for random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting, was created. Quantitative assessment of the quality of the RCTs was performed using a modified Jadad 7-point scale, where a Jadad score ≥ 4 indicates high-quality [12]. The overall quality of each study was evaluated as “low” or “high.” Publication bias was assessed using a funnel plot when the number of included studies was ≥ 10 [13].
Statistical analysis
Review Manager software version 5.4 (Cochrane Collaboration, England) was used for this meta-analysis. The incidence of pulmonary complications was a dichotomous outcome, while the remaining outcomes were continuous. Odds ratios (ORs) and 95% CIs were used to assess dichotomous outcomes, while mean differences (MDs) and 95% CIs were used to assess continuous outcomes. The length of hospital stay and other continuous outcomes were assessed based on the difference between the value at the observational time point and the value before drug treatment. A meta-analysis was performed when an outcome was reported in two or more studies. Statistical heterogeneity among the included studies was assessed using P and I2. A fixed-effects model was applied when I2 < 50% and P > 0.1; otherwise a random-effects model was used. The inverse variance and Mantel-Haenszel methods were used to combine separate statistics. Statistical significance was set at P < 0.05. A sensitivity analysis was conducted by omitting one study in turn.
Trial sequential analysis (TSA) software version 0.9.5.10 (Copenhagen Trial Unit, Denmark) was used to examine the reliability and conclusiveness of the available evidence according to a previous meta-analysis [14,15]. A sufficient level of evidence was determined to have been reached for the anticipated intervention effect when the cumulative Z-curve crossed the TSA boundary and no further studies were needed. In contrast, when the Z-curve failed to cross the TSA boundary and the required information size (RIS) was not reached, the evidence was considered insufficient to reach a conclusion. Two-sided tests with a type I error of 5%, power of 80%, and low bias-based relative risk reduction were used to calculate the RIS.
Results
Search results
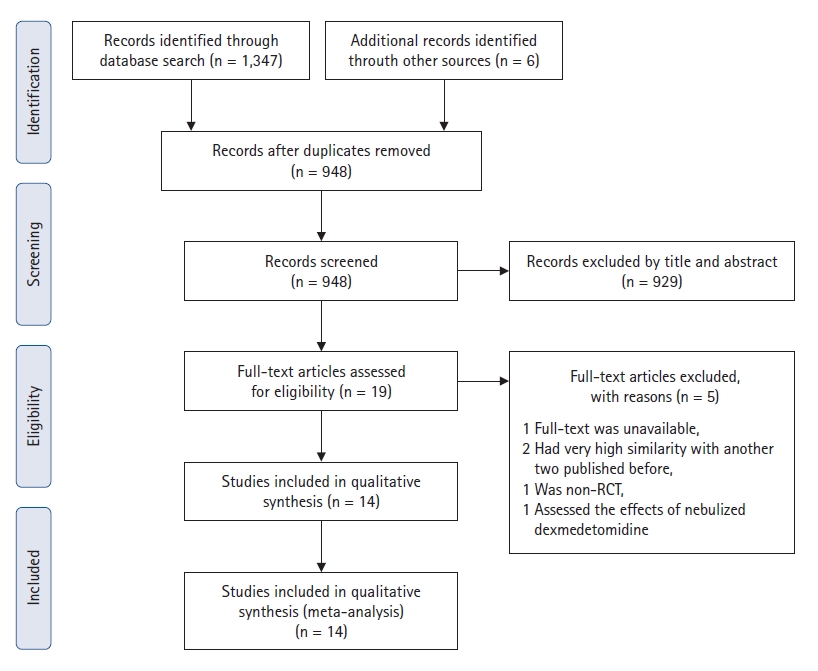
A total of 948 studies were identified, of which 929 were excluded after screening the titles and abstracts (Fig. 1). After screening the full text of the remaining 19 articles, one study published in 2017 [16] was excluded because it was extremely similar to another study published in 2016 [17]. Two studies by Xia et al. [18,19] reached the same conclusion; therefore, we only included the latest study [19]. Among the remaining 17 studies, three were excluded for the following reasons: one was not an RCT [20], one had no full text available [21], and one assessed the effect of nebulized dexmedetomidine [22]. Thus, 14 RCTs [1,8,9,17,19,23–31], with 845 total patients, were included in this meta-analysis.
The basic characteristics and interventions are summarized in Supplementary Table 1. All RCTs were published after 2010. One RCT [28] was published in the USA, whereas all remaining RCTs were published in Asia. In one RCT [24], dexmedetomidine was intravenously infused at a rate of 0.3 μg/kg/h, whereas, in the remaining RCTs, it was infused as a bolus dose of 0.3–1.0 μg/kg over 10 min and then as a continuous infusion at 0.3–0.5 μg/kg/h. Two RCTs [1,25] investigated different doses of dexmedetomidine.
Risk of bias assessment
The quality of the RCTs was assessed using the risk of bias and modified Jadad scores (Table 1). Five RCTs [1,9,19,25,31] did not provide details regarding randomization. Five RCTs [8,17,19,25,28] reported the implementation of allocation concealment using sealed envelopes. Seven RCTs [8,9,17,19,25,27,28] reported the use of patient and participant blinding. None of the studies reported blinding of the outcome assessment. Eight RCTs [1,24,26–31] did not report the number and reasons for patient withdrawal or loss to follow-up; therefore, we determined that these studies had incomplete outcome data. The PaO2 results in one RCT [1] were inconsistent with the data shown in the table; therefore, we concluded that the study selectively reported the outcomes. None of the other sources of bias were applicable. Eight RCTs [8,9,17,19,23,25,27,28] with a modified Jadad score ≥ 4 were rated as high quality.
Meta-analysis results
Oxygenation index
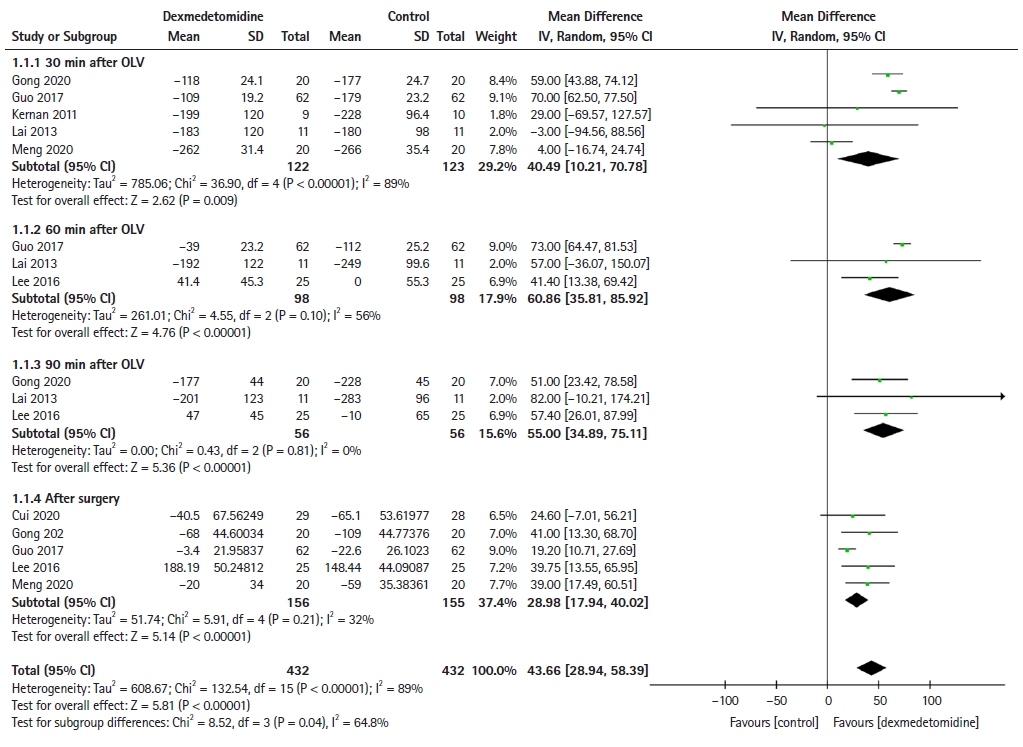
Five [24,26,28,29,31], three [17,26,29], three [17,24,29], and five [17,23,24,26,31] RCTs reported the OI at 30, 60, and 90 min after OLV and after surgery, respectively. Although the OI decreased in both the control and dexmedetomidine groups after OLV (Fig. 2), dexmedetomidine significantly improved the OI at 30 min (MD: 40.49, 95% CI [10.21, 70.78], P = 0.009), 60 min (MD: 60.86, 95% CI [35.81, 85.92], P < 0.001), and 90 min (MD: 55, 95% CI [34.89, 75.11], P < 0.001) after OLV and after surgery (MD: 28.98, 95% CI [17.94, 40.02], P < 0.001) compared with the control group.
Respiratory mechanics
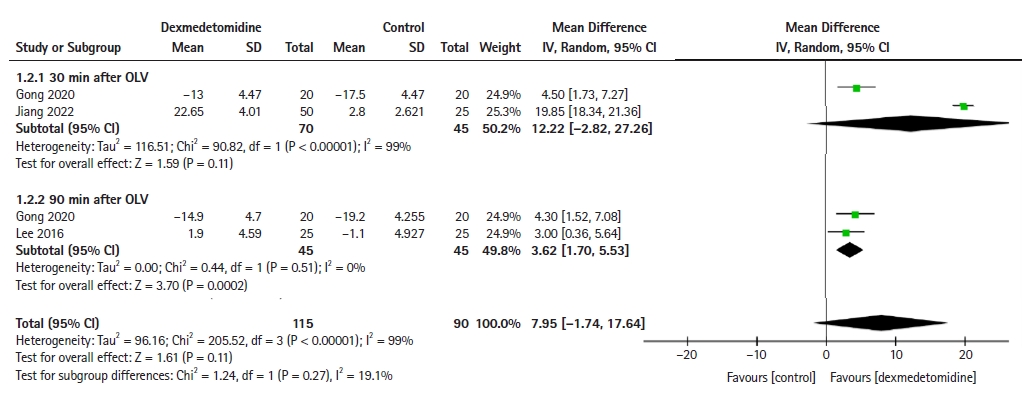
The following indices were used to assess respiratory mechanics: lung compliance, Pplat, Ppeak, airway resistance, dead space ventilation, transpulmonary pressure, and Qs/Qt. However, only lung compliance, Pplat, and Qs/Qt were included in the meta-analysis. Two [1,24] and two [17,24] RCTs reported lung compliance at 30 and 90 min after OLV, respectively. Although dexmedetomidine did not improve lung compliance 30 min after OLV (MD: 12.22, 95% CI [−2.82, 27.26], P = 0.11), compliance improved significantly 90 min after OLV (MD: 3.62, 95% CI [1.7, 5.53], P < 0.001) compared with the control group (Fig. 3). Two RCTs [1,26] reported Pplat and three studies [1,19,25] reported Qs/Qt 30 min after OLV. The meta-analysis also showed that dexmedetomidine did not decrease the Pplat (MD: −10.41, 95% CI [−25.56, 4.73], P = 0.18; Supplementary Fig. 1) or Qs/Qt (MD: −7.45, 95% CI [−24.88, 9.79], P = 0.40; Supplementary Fig. 2) 30 min after OLV compared with the control group.
Serum inflammatory factors
Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8 were integrated to evaluate the effects of dexmedetomidine on inflammatory reactions. Three [1,8,26], two [26,30], and four [8,23,26,30] RCTs reported the TNF-α levels at 30 min after OLV, 60 min after OLV, and after surgery, respectively. This meta-analysis showed that dexmedetomidine decreased the TNF-α levels significantly 30 min after OLV (MD: -20.38, 95% CI [−34.84, -5.92], P = 0.006) and after surgery (MD: −19.67, 95% CI [−34.51, −4.83], P = 0.009), but not 60 min after OLV (MD: −25.31, 95% CI [−54.48, 3.85], P = 0.09) compared with the control group (Supplementary Fig. 3). Three [1,8,24] and four [8,23,24,30] RCTs reported the level of IL-6 30 min after OLV and after surgery, respectively. Although the IL-6 levels were not significantly decreased 30 min after OLV (MD: −13.62, 95% CI [−34.48, 7.23], P = 0.2), they were significantly decreased after surgery (MD: −5.52, 95% CI [−8.00, −3.04], P < 0.001) in the dexmedetomidine group compared with the control group (Supplementary Fig. 4). Two RCTs [1,31] reported IL-8 levels 30 min after OLV. This meta-analysis found that dexmedetomidine significantly decreased the level of IL-8 30 min after OLV (MD: −37.57, 95% CI [−41.91, −33.24], P < 0.001; Supplementary Fig. 5) compared with the control group.
Serum oxidative stress indices
Malondialdehyde (MDA) and superoxide dismutase (SOD) were integrated to evaluate the effects of dexmedetomidine on oxidative stress reactions. Four [1,19,24,26], two [26,30], and three [24,26,30] RCTs reported MDA levels 30 min after OLV, 60 min after OLV, and after surgery, respectively. This meta-analysis found that dexmedetomidine greatly decreased the MDA levels at 30 min (MD: −3.47, 95% CI [−5.17, −1.78], P < 0.001) and 60 min (MD: −0.45, 95% CI [−0.81, −0.08], P = 0.02) after OLV and after surgery (MD: −0.58, 95% CI [−0.98, −0.17], P = 0.006) compared with the control group (Supplementary Fig. 6). Three [1,19,24] and two [24,30] RCTs reported MDA levels at 30 min after OLV and after surgery, respectively. Although SOD levels were not significantly increased 30 min after OLV (MD: 8.34, 95% CI [−3.62, 20.3], P = 0.17), they were significantly increased after surgery (MD: 29.07, 95% CI [22.01, 36.13], P < 0.001) in the dexmedetomidine group compared with the control group (Supplementary Fig. 7).
Hemodynamic indices
HR and MAP were integrated to evaluate the effects of dexmedetomidine on hemodynamic indices. Six RCTs [1,9,19,25,28,31] reported the HR and MAP values 30 min after OLV, and three RCTs [9,17,25] reported the HR and MAP values 60 min after OLV. This meta-analysis showed that dexmedetomidine did not significantly decrease HR at 30 min (MD: −2.13, 95% CI [−4.30, 0.04], P = 0.05) or 60 min (MD: −10.09, 95% CI [−20.48, 0.30], P = 0.06) after OLV compared with the control group (Supplementary Fig. 8). Additionally, dexmedetomidine did not significantly decrease MAP at 30 min (MD: −1.89, 95% CI [−3.81, 0.04], P = 0.05) or 60 min (MD: −10.25, 95% CI [−22.01, 1.51], P = 0.09) after OLV compared with the control group (Supplementary Fig. 9).
Postoperative pulmonary complications
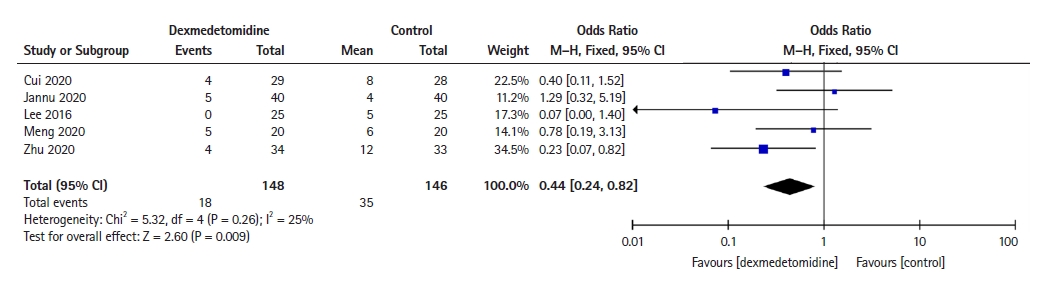
Five RCTs [8,17,23,27,31] reported the incidence of postoperative pulmonary complications, including pulmonary infection, atelectasis, pneumonia, acute respiratory distress syndrome, purulent sputum, prolonged air leakage, and pulmonary embolism. This meta-analysis showed that dexmedetomidine significantly decreased the incidence of postoperative pulmonary complications (OR: 0.44, 95% CI [0.24, 0.82], P = 0.009; Fig. 4) compared with the control group.
Length of hospital stay
Four RCTs [8,17,27,31] reported the length of hospital stay. This meta-analysis showed that dexmedetomidine significantly decreased the length of hospital stay (MD: −0.99, 95% CI [−1.25, −0.73], P < 0.001; Fig. 5) compared with the control group.
Sensitivity analysis
The sensitivity analysis revealed that significant differences in the OI at 60 and 90 min after OLV and after surgery, IL-6 levels after surgery, and length of hospital stay between the dexmedetomidine and control groups persisted when one study was omitted in turn (Supplementary Table 2). The other outcome variables either showed no differences or sensitivity analyses could not be performed as only two RCTs were included.
TSA
The TSA showed that the Z-curves of the OI (Supplementary Fig. 10) and IL-6 levels (Supplementary Fig. 11) after surgery crossed the conventional and TSA boundaries, and the Z-curve of the length of hospital stay (Supplementary Fig. 12) crossed the conventional boundary but did not cross the TSA boundary.
Discussion
As this study aimed to assess the effects of dexmedetomidine on pulmonary function in operative patients receiving mechanical ventilation, the primary protocol of this meta-analysis was intended to include different types of surgery. However, after comprehensively searching the databases, we found several related studies that included patients undergoing OLV. To reduce bias, we adjusted the inclusion criteria to only include patients who underwent OLV. Our meta-analysis showed that dexmedetomidine infusion significantly improved the OI at 30, 60, and 90 min after OLV and after surgery. These results were confirmed using sensitivity analysis and TSA, and were consistent with two previous meta-analyses published by Bai et al. [2] and Huang et al. [32], which showed that intraoperative dexmedetomidine treatment improved oxygenation in patients receiving OLV. In addition, studies have reported that nebulized dexmedetomidine treatment improves PaO2 during OLV [22] and intravenous dexmedetomidine treatment improves oxygenation both in morbidly obese patients undergoing bariatric surgery [33] and in patients with cervical cancer undergoing laparoscopy [34]. Taken together, these data strongly suggest that intraoperative dexmedetomidine treatment improves oxygenation in patients receiving mechanical ventilation.
Similar to the findings of the meta-analysis by Bai et al. [2], the current study also found that perioperative dexmedetomidine administration decreased the serum concentrations of TNF-α, IL-6, and IL-8 in patients receiving OLV. However, the anti-inflammatory effect of dexmedetomidine could not completely explain the increase in the OI at 30 min after OLV. As respiratory mechanics directly affect oxygenation, we evaluated the effects of dexmedetomidine on respiratory mechanics. This meta-analysis found that dexmedetomidine infusion significantly improved lung compliance 90 min after OLV. Although our study found that dexmedetomidine had no effect on lung compliance, Pplat, or intrapulmonary shunt 30 min after OLV, a limited number of RCTs were included. Moreover, although one meta-analysis [32] found that intraoperative dexmedetomidine treatment reduced the intrapulmonary shunt level during OLV, only five studies were included in the analysis, two of which were conducted by the same authors. Lee et al. [17] reported that intraoperative dexmedetomidine treatment decreased the Ppeak in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery. Jannu et al. [27] found that intraoperative dexmedetomidine treatment improved the forced expiratory volume in 1 s on postoperative days 1 and 2. Another retrospective study [35] found that intraoperative dexmedetomidine treatment reduced the Ppeak and airway resistance at the end of OLV. To the best of our knowledge, no previous meta-analysis has investigated the effects of dexmedetomidine on oxidative stress reactions. Our findings revealed that intraoperative dexmedetomidine treatment decreased serum MDA levels and increased serum SOD levels; however, the number of RCTs included was limited. Thus, although dexmedetomidine has the potential to improve respiratory mechanics and regulate oxidative stress, more high-quality RCTs are required to confirm these findings.
One concern of intraoperative dexmedetomidine treatment is cardiovascular side effects. However, our meta-analysis found no significant differences in HR or MAP between the dexmedetomidine and control groups. Similarly, one previous meta-analysis [36], which included 10 RCTs, showed that intraoperative dexmedetomidine infusion had little effect on MAP and HR during bariatric surgery. Although some meta-analyses [32,37] found that dexmedetomidine decreased the perioperative MAP and HR, others [38,39] found that perioperative dexmedetomidine infusions resulted in more stable hemodynamics. These results suggest that dexmedetomidine infusions are not associated with severe cardiovascular side effects.
Finally, we found that intraoperative dexmedetomidine treatment reduced the length of hospital stay and the incidence of postoperative pulmonary complications. Although the sensitivity analysis and TSA revealed these results to be inconclusive, they were highly consistent with those of other meta-analyses [36,40]. Based on the results, the mechanisms by which dexmedetomidine improves oxygenation and pulmonary function during OLV can be speculated. Dexmedetomidine inhibits lung inflammation, regulates oxidative stress, and improves lung compliance, leading to reduced alveolar edema, increased pulmonary gas exchange, and enhanced alveolar ventilation. Furthermore, improving oxygenation and pulmonary function decreases the incidence of postoperative pulmonary complications and the length of hospital stay. Considering the numerous advantages of dexmedetomidine and the lack of severe cardiovascular side effects, we recommend that dexmedetomidine be routinely used in patients receiving mechanical ventilation.
This meta-analysis had some limitations. First, except for the OI and IL-6 levels, we were unable to draw definitive conclusions regarding the remaining outcomes as the sample sizes were small and some studies were poorly designed. Second, the different starting times and doses of dexmedetomidine may have affected the results. Lastly, several of the included studies were performed in Asia, especially in China, and thus geographical limitations are present. As we know, authors in the same region may has more opportunities to communicate, thus, their results may be affected by each other. Moreover, it is unclear whether the results of this meta-analysis are suitable for patients in other regions, such as Europe and Africa.
Overall, intraoperative dexmedetomidine treatment improves oxygenation in patients receiving OLV and may decrease the incidence of postoperative pulmonary complications and shorten the length of hospital stay, which may be related to associated improvements in lung compliance, anti-inflammatory effects, and regulation of oxidative stress reactions. However, robust evidence is required to confirm these conclusions.
Notes
Funding
This work is supported by Grant [2021]24 from the Kuanren Talents Program of the Second Affiliated Hospital of Chongqing Medical University.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author Contributions
Lin Yang (Data curation; Formal analysis; Investigation; Software; Writing – original draft)
Yongheng Cai (Data curation; Formal analysis; Investigation; Software; Writing – original draft)
Lin Dan (Supervision; Validation; Visualization; Writing – review & editing)
He Huang (Supervision; Validation; Visualization; Writing – review & editing)
Bing Chen (Conceptualization; Funding acquisition; Supervision; Writing – original draft; Writing – review & editing)
Supplementary Materials
Search strategy.
Characteristics of the included studies.
Forest plot diagram showing Pplat. SD: standard deviation, IV: inverse variance, OLV: one-lung ventilation.
Forest plot diagram showing Qs/Qt. SD: standard deviation, IV: inverse variance, OLV: one-lung ventilation.
Forest plot diagram of TNF-α levels. SD: standard deviation, IV: inverse variance, OLV: one-lung ventilation.
Forest plot diagram of IL-6 levels. SD: standard deviation, IV: inverse variance, OLV: one-lung ventilation.
Forest plot diagram of IL-8 levels. SD: standard deviation, IV: inverse variance, OLV: one-lung ventilation.
Forest plot diagram showing MDA levels. SD: standard deviation, IV: inverse variance, MDA: malondialdehyde, OLV: one-lung ventilation.
Forest plot diagram showing SOD levels. SD: standard deviation, IV: inverse variance, SOD: superoxide dismutase, OLV: one-lung ventilation.
Forest plot diagram showing HR. SD: standard deviation, IV: inverse variance, HR: heart rate, OLV: one-lung ventilation.
Forest plot diagram showing MAP. SD: standard deviation, IV: inverse variance, MAP: mean arterial pressure, OLV: one-lung ventilation.
Sensitivity analysis of positive outcomes.
Sequential trial analysis of the OI after surgery. RIS: required information size. OI: oxygenation index.
Sequential trial analysis of IL-6 levels after surgery. RIS: required information size.
Sequential trial analysis of length of hospital stay. RIS: required information size.