 |
 |
| Korean J Anesthesiol > Volume 76(6); 2023 > Article |
|
Abstract
Background
Methods
Results
NOTES
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Ah Ran Oh (Conceptualization; Data curation; Formal analysis; Validation; Writing – original draft)
Jungchan Park (Conceptualization; Formal analysis; Supervision; Writing – original draft; Writing – review & editing)
Jong-Hwan Lee (Supervision; Writing – review & editing)
Kwangmo Yang (Software; Supervision; Validation)
Joonghyun Ahn (Formal analysis)
Seung-Hwa Lee (Supervision; Validation; Writing – review & editing)
Sangmin Maria Lee (Supervision; Writing – review & editing)
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Fig. 1.
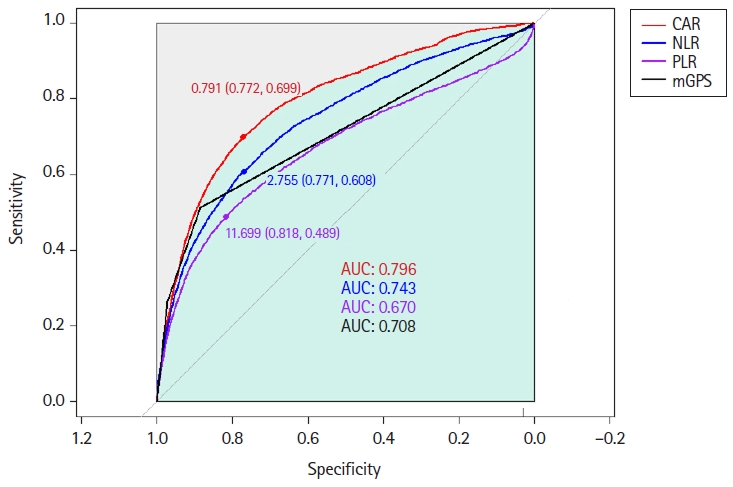
Fig. 2.
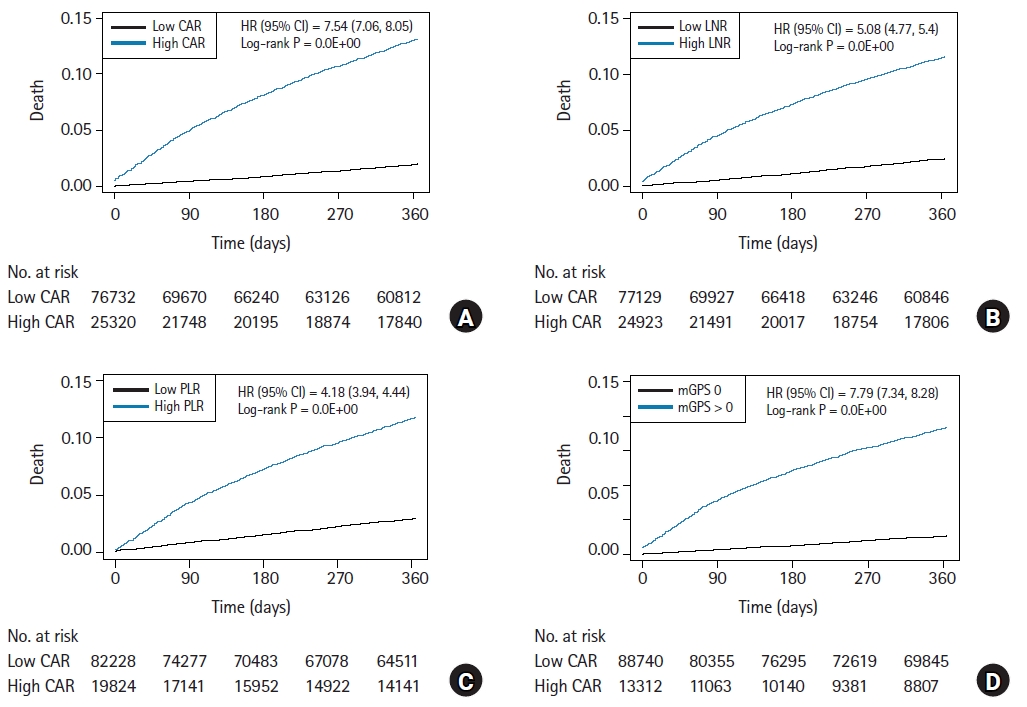
Table 1.
Values are presented as median (Q1, Q3), number (%) or mean ± SD. Surgical risk was stratified according to the 2014 European Society of Cardiology/European Society of Anesthesiology guidelines. CAR: CRP-to-albumin ratio, NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, mGPS: modified Glasgow Prognostic Score, CRP: C-reactive protein, ASA: American Society of Anesthesiologists.









