Introduction
Pacemaker implantation in children requires general anesthesia. However, for a child with complete heart block, anesthetic challenges can include bradycardia and hypotension unresponsive to conventional drugs and directly proportional to the depth of anesthesia [
1].
Owing to the rich innervation of the chest wall in younger children, perioperative and postoperative analgesia after transvenous pacemaker placement presents unique challenges. The most stimulating parts of subpectoral pacemaker insertion include the initial incision and the expansion of the generator pocket, both of which require either an increase in the depth of anesthesia or the use of an appropriate regional anesthesia technique [
2].
Postoperative pain management routinely involves either intravenous or oral opioid agents in addition to nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol. To minimize postoperative narcotic use, interest in opioid-sparing multimodal pain management in the pediatric population has increased [
3].
Regional anesthesia is an integral component of this era of multimodal analgesia and enhanced recovery after surgery in adult and pediatric populations [
4]. Additionally, the use of ultrasound guidance in regional anesthesia has dramatically improved routine pediatric perioperative pain management [
5]. Pectoral nerve (PECS) blocks were first described by Blanco in 2011 [
6]. PECS blocks are novel ultrasound-guided fascial plane blocks intended to provide anesthesia and/or analgesia of the upper anterior chest wall without the more serious complications associated with neuraxial techniques or paravertebral blocks [
7].
As very few studies have investigated outcomes associated with PECS blocks in children, we designed this prospective randomized controlled trial to evaluate the effects of PECS blocks versus standard treatment on perioperative pain control and opioid consumption in children undergoing transvenous subpectoral pacemaker insertion.
Materials and Methods
Trial design
This prospective, single-center, parallel-group, randomized controlled trial with quintuple-blinded (patients, parents, cardiologists, intensive care unit [ICU] staff, and evaluators), parallel-group randomized controlled trial was conducted in a cardiac catheterization laboratory at Aboelriesh pediatric hospital.
Ethical approval and clinical trial authorization
This study received approval from the ethical committee board of Kasr Alainy school of medicine (approval number: MS-316-2020) on April 8, 2021. Prior to participant enrollment, the trial was registered at ClinicalTrials.gov on June 17, 2021 (registration number: NCT04931693). The protocol was written according to the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement guidelines, adhered to the Standard Protocol Items: Statement of Recommendations for Interventional Trials (SPIRIT), and was carried out according to the current version of the Helsinki Declaration-2013 and Egyptian law on the protection of personal information. The patients’ legal guardians provided written informed consent before participation in the study.
Study population
We enrolled 40 children of both sexes, aged 1 to 9 years, who underwent transvenous subpectoral pacemaker insertion with either congenital or postoperative complete heart block, as described in the flow diagram (
Fig. 1).
Patients with known coagulopathy, a rash or signs of infection at the injection site, known allergy to local anesthesia, previous subpectoral pacemaker insertion, or emergency procedure were excluded from the study.
Intervention
Patients who met all the inclusion criteria were randomly allocated to either the control (Group C) or pectoral (Group P) group. In Group C, analgesics were administered intravenously to control perioperative pain, while in Group P, participants received 0.25% bupivacaine between the pectoralis major and minor muscles (PECS block type I) and below the pectoralis minor and above the serratus anterior muscles (PECS block type II) at a volume of 0.5 ml/kg divided equally between the two sites (0.25 ml/kg at each site).
Randomization and blinding
An online randomization program (
http://www.randomizer.org) was used to generate a random list, and patients were randomly allocated at a ratio of 1 : 1 into either Group P (n = 20) or Group C (n = 20). The random allocation numbers were concealed in closed opaque envelopes.
Anesthesia and perioperative care
Complete histories, comprehensive clinical examinations, complete blood counts, coagulation profiles, and CRP levels as well as preoperative electrocardiography (ECG), echocardiography, and chest radiography were all conducted as part of the preoperative anesthetic evaluations. Children were given intramuscular both atropine 0.01 mg/kg and midazolam 0.02 mg/kg 20 min before being admitted to the operating room.
Upon arrival at the operating room, ECG, pulse oximetry (SpO2), non-invasive arterial blood pressure, temperature, end-tidal CO2, and transcutaneous pacemaker pads were applied, and baseline readings were obtained before general anesthesia was induced with 8 vol% sevoflurane and a 50% oxygen in air. Following loss of consciousness, a peripheral venous cannula was inserted. For patients with an intravenous cannula already in place, 1.5 mg/kg propofol was used for induction. In both cases, 0.5 mg/kg intravenous atracurium was administered to facilitate endotracheal intubation and 2 µg/kg fentanyl was administered to eliminate the stress response associated with intubation. The patients’ lungs were ventilated with 50% oxygen in air at tidal volumes of 6–8 ml/kg, and the respiratory rate was adjusted to maintain the end-tidal carbon dioxide concentration at 30–35 mmHg. Anesthesia with 1% isoflurane, 0.1 mg/kg atracurium, and 0.50 µg/kg fentanyl were administered to maintain blood pressure and heart rate [HR] below 120% of baseline values.
Patient positioning and preparation for the block
A consultant regional anesthetist performed the ultrasound-guided PECS block for the children allocated to Group P. The chest was first prepared in a sterile fashion, and a linear ultrasound probe (S-Nerve
TM; SonoSite Inc., USA) was placed on the surgical side for pacemaker placement at the level of the third rib. The pectoralis major, pectoralis minor, and serratus anterior muscles were identified. A 22 gauge needle was advanced from the anteromedial to posterolateral direction using an in-plane technique until the fascial plane was reached, and 0.25 ml/kg of 0.25% bupivacaine was administered between the pectoralis major and minor muscles (PECS block I). The probe was moved laterally to identify the fourth rib. The needle was then advanced to administer another 0.25 ml/kg 0.25% bupivacaine between the pectoralis minor and serratus anterior muscles (PECS block II) (
Fig. 2) [
8].
Pacemaker placement
All procedures were performed by a pediatric electrophysiologist. Following the PECS block, the chest was reprepared and draped in a sterile manner, and 10 ml of contrast was injected under fluoroscopy through peripheral lines in the right and left arms to delineate the right and left subclavian veins. A 5-cm incision was made just below the clavicle in the left upper chest, and electrocautery was used to dissect the prepectoral fascia. A pocket was created under the pectoral muscle using blunt dissection. A micropuncture needle was used to gain access to the subclavian vein, which was then dilated to a larger introducer-sheath size. The pacemaker lead was then advanced through the sheath to a suitable location on the heart. After correct positioning was confirmed, the lead was connected to the generator and attached to the pectoral muscle. The device was placed in the pocket after sufficient irrigation with gentamicin-infused saline. Three distinct layers were formed by closing the fascial, subcutaneous, and superficial skin layers.
Since the implanted pacemakers were all rate-responsive, meaning the HR can increase in response to increased physical needs, vital signs (HR and non-invasive blood pressure) were recorded at the start of the block, every 15 min intraoperatively, and every 6 h postoperatively for 24 h.
After skin closure, inhalational anesthesia was discontinued and muscle relaxation was reversed after the return of spontaneous breathing. The patients were extubated and transferred to the pediatric intensive care unit (PICU) for recovery and monitoring.
Postoperative assessment and analgesia regimen
After admission to the PICU, patients in both groups were managed according to the PICU protocol, which includes standard monitoring and intravenous analgesic paracetamol 7.5 mg/kg every 6 h. The Face, Legs, Activity, Cry, Consolability scale (FLACC) was assessed immediately post-operation in the PICU and every 6 h for 24 h (
Table 1) [
9]. When the FLACC scale score was ≥ 4 at rest, rescue analgesia, which comprised incremental intravenous morphine at a dose of 0.1 mg/kg (with a maximum single dose of 0.1 to 0.2 mg/kg/dose repeated every 60 min according to the patients’ response), was administered to maintain a resting FLACC scale score < 4. Cumulative 24-h analgesic consumption was recorded [
10].
Data collection
Data were collected independently by a researcher who was not aware of the treatment assignment and was not involved in clinical care decision-making. The baseline characteristics included age, sex, weight, and cause of complete heart block. Intraoperative data included the duration of surgery, duration of the block, hemodynamic parameters (HR, mean arterial pressure), and cumulative fentanyl and atracurium doses. Postoperative data included the postoperative pain score, time to first rescue analgesia, postoperative morphine consumption, and postoperative complications (postoperative nausea and vomiting, pneumothorax, and infection).
Study outcomes
The primary outcome of this study was the mean pain score based on the FLACC scale in the first 24 h. Secondary outcomes were as follows: time to first postoperative rescue analgesia, total opioid consumption, total dose of muscle relaxants, perioperative hemodynamic parameters, and incidence of postoperative complications.
Statistical considerations
Sample size
The sample size was calculated using the MedCalc Software version 14 (MedCalc Software bvba, Belgium). We performed a pilot study of six patients in the control group and the mean pain score in the first 24 h was found to be 3 ± 0.57 (mean ± standard deviation [SD]). With an alpha error of 0.05, we calculated that 36 children would provide a power of 90% to detect a 20% (0.6) difference in the mean pain score in the first 24 h between the two groups. To compensate for dropouts, we increased the required sample size to 40 patients (20 patients per group).
Data analysis
All statistical comparisons were performed using the Statistical Package for Social Science (SPSS), version 21 (USA). The mean and SD were used to express continuous quantitative normally distributed data, whereas the median and range were used to express non-normally distributed data. Percentages were used to express qualitative nominal data. Following normality testing, continuous variables were compared using the t-test or Mann-Whitney U test, as appropriate. The chi-square test or Fisher’s exact test was used to compare categorical variables. Analysis of variance (ANOVA) for repeated measures with Bonferroni correction was used to compare the changes over time. Statistical significance was set at P < 0.05.
Results
Study population
Forty children (20 in each group) met the inclusion criteria and were enrolled in this study. Group C (control) received conventional analgesics without any block, while Group P (pectoral) received PECS blocks. None of the patients were excluded from the study (
Fig. 1). There were no statistically significant differences in baseline characteristics between the two groups (
Table 2).
Primary outcome
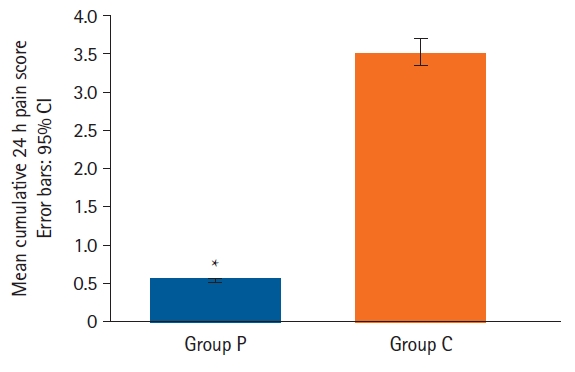
The mean pain score was significantly lower in the pectoral group compared to the control group (mean values [95% CI] for Group P and Group C were 0.54 [0.40, 0.73] and 3.52 [3.16, 3.72], respectively) (P < 0.001) (
Table 3 and
Fig. 3).
Secondary outcomes
For the intraoperative variables, the mean duration of surgery was significantly longer in Group P than in Group C (P = 0.032). However, the mean cumulative doses of fentanyl and atracurium were also significantly lower in Group P than in Group C (P = 0.040 and P < 0.001, respectively) (
Table 2).
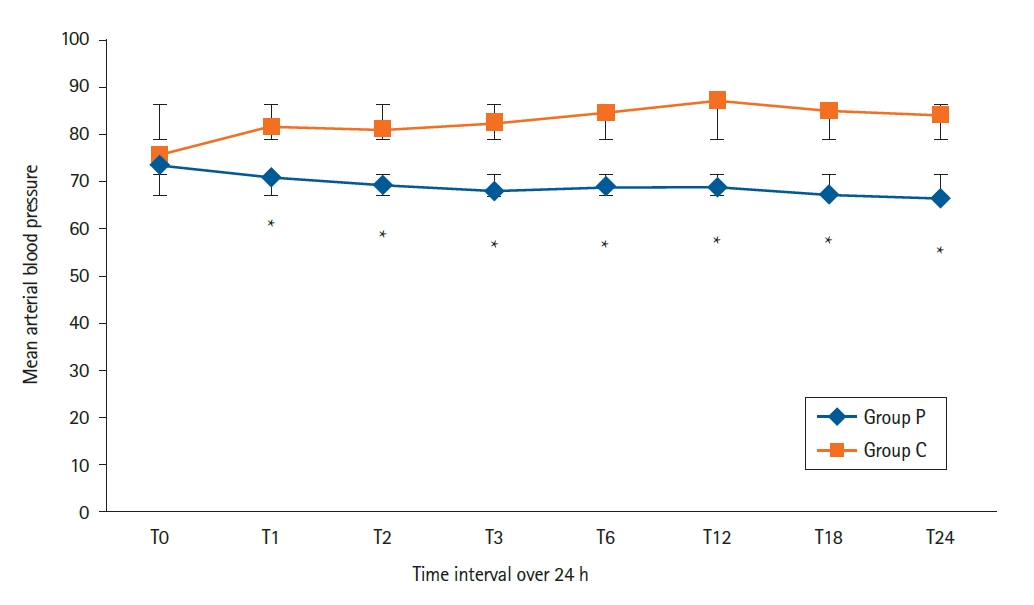
Hemodynamic parameters (mean arterial pressure and HR) were measured at the following time points: baseline (T0), skin incision (T1), every 15 min until extubation, every 6 h following extubation. Our study showed no significant differences between the two studied groups at T0 (P = 1.00). However, a statistically significant difference was seen at each time point from T1 to T24 (P < 0.036), with higher values in the control group than the pectoral group (
Fig. 4).
For postoperative variables, the postoperative FLACC pain scale score, compared to the control group, the pectoral group experienced significantly less pain at all time points (
Table 3). The postoperative mean cumulative morphine dose was significantly lower in Group P than in Group C (P = 0.022) (
Table 2).
The first request for rescue analgesia occurred later in the pectoral group than in the control group, with a median time of 2 h and 7 h in the control and pectoral groups, respectively (
Table 2).
There were no statistically significant differences in the incidence of complications, such as PONV, pneumothorax, or infection, between the two groups (
Table 2).
Discussion
This randomized controlled study aimed to evaluate the differences in perioperative pain control and opioid consumption in the first 24 h provided by ultrasound-guided PECS blocks versus standard postoperative pain control in children undergoing transvenous subpectoral pacemaker insertion.
All device implants in pediatric patients are performed under general anesthesia, and many of these patients have high-risk cardiorespiratory comorbidities that make general anesthesia more dangerous [
8]. Maintaining effective anesthesia and hemodynamic stability are important goals during pacemaker insertion in pediatric patients with complete heart blocks because the risk of vascular tone loss and diminishing cardiac output during the procedure is always present. Therefore, induction and maintenance of general anesthesia in patients with complete heart blocks must be performed very carefully [
1]. Additionally, the rich innervation of the chest wall in younger children undergoing subpectoral placement of pacemakers can lead to greater pain from the dissection of pectoralis muscle fibers [
2].
Adequate postoperative analgesia for cardiac procedures aids in early recovery and ambulation and reduces postoperative complications. Pain in post-intervention cardiac patients is typically treated with NSAIDs, paracetamol, and opioids, which are associated with numerous side effects and delayed recovery [
11].
Growing awareness of the risks associated with narcotic use in pediatric and adolescent populations has prompted researchers to investigate opioid-free postoperative pain management with regional anesthesia. The PECS blocks I and II were introduced in 2011 by Blanco [
6] for analgesia in patients undergoing breast surgery and have since been adapted for various chest wall surgeries, such as thoracotomy and device placement in adults.
As the risk of infection is always present with hardware placement, we were concerned about re-preparation of the surgical site between the PECS block and device placement because they are in the same location. In our current practice, PECS blocks are performed using a sterile technique, followed by additional surgical preparation to ensure a sterile field before the first incision for device implantation. Our data revealed a statistically significant increase in operating room and procedural times in the pectoral group. We discussed the possibility of eliminating the additional surgical site preparation, as this could reduce the operating room time.
Relevant case reports have evaluated device implantation under PECS blocks in adults, including a PECS block with an intercostal nerve block and intravenous sedation for the subpectoral placement of a cardiac resynchronization therapy device [
12], a PECS block with an intercostal block and intravenous sedation for implantable cardioverter defibrillator (ICD) insertion in young patients with Duchenne muscular dystrophy [
13], a PECS block for ICD insertion in a morbidly obese patient [
14], relocation of an infected cardiac pacemaker generator under a PECS block using intravenous midazolam [
15], and a PECS block alone as the primary anesthetic technique without any sedation or intravenous anesthetics [
16]. All studies found that the PECS block provided effective analgesia.
Our findings are consistent with those of Yang et al. [
8], who evaluated PECS blocks in pediatric patients undergoing transvenous pacemaker or ICD placement between January 2015 and August 2020 at Lucile Packard Children’s Hospital–Stanford University. Based on data on 20 patients undergoing PECS blocks, the authors concluded that these blocks reduced postoperative pain scores and lowered total opioid consumption after ICD or pacemaker placement and they should be considered for transvenous device placement in children.
Our study had some limitations. First, it was a single-center study with a small sample size determined by the primary outcome and thus drawing definitive conclusions about complications was difficult. Second, PECS blocks were not used as the sole anesthetic technique but as an adjunct to general anesthesia. Third, this study was limited to a follow-up period of 24 h due to early discharge to home.
However, to the best of our knowledge, this is the first randomized controlled study to evaluate the use of PECS blocks in pediatric patients undergoing transvenous subpectoral pacemaker placement. Further studies in this context with a larger sample size and longer follow-up are needed to confirm our findings.
The ultrasound-guided PECS block is a relatively easy fascial block technique to perform with a good intraoperative hemodynamic profile, reduced postoperative pain scores, and lower total opioid consumption in children undergoing transvenous subpectoral pacemaker placement. Therefore, PECS blocks should be considered when transvenous subpectoral pacemakers are placed in children.