Current evidence of ultrasound-guided fascial plane blocks for cardiac surgery: a narrative literature review
Article information
Abstract
Fascial plane blocks are useful for multimodal analgesia after cardiac surgery since they can provide effective analgesia without the serious risks associated with conventional techniques such as neuraxial hematoma and pneumothorax. This narrative review covers blocks performed at the parasternal intercostal, interpectoral, pectoserratus, serratus anterior, erector spinae, and retrolaminar planes, which are targets for fascial plane blocks in cardiac surgery. Brief anatomical considerations, mechanisms, and currently available evidence are reviewed. Additionally, recent evidence on fascial plane blocks for subcutaneous-implantable cardioverter-defibrillator implantation are also reviewed.
Introduction
Cardiac surgeries are mainly performed via sternotomy and have been conventionally managed using high doses of opioids to attenuate undesirable physiologic responses [1]. However, such high opioid doses are associated with unwanted risks such as prolonged mechanical ventilation and a longer intensive care unit or hospital stay. These practices have recently been changing with the introduction of concepts of enhanced recovery after surgery [2] and fast-tracking [3,4], which aim to achieve both quick and high-quality recovery. As these goals can conflict with a high-dose opioid regimen, perioperative analgesic protocols should ideally involve alternatives or supplements for opioids. Effective pain control contributes not only to an improvement in patient comfort and satisfaction but also clinical outcomes [5–8].
Cardiac surgery has become increasingly less invasive with advancements in surgical instruments and techniques, such as minimal invasive direct coronary artery bypass (MIDCAB) graft surgery and anterior thoracotomy for valve surgery [9]. As the rationale for applying these less invasive techniques is to alleviate physiological deterioration due to extensive surgical wounds and accompanying pain and thereby enhance recovery, immediate or early extubation is often attempted in these patients. In contrast to classical cardiac surgical patients who were heavily sedated with high doses of sedatives and opioids in the early postoperative period, these patients have to confront this stressful time without the assistance of mechanical ventilation and with minimal use of sedatives and opioids. To effectively accomplish this, numerous regional analgesic techniques have been introduced as essential components of an effective multimodal analgesic protocol [10–16].
Among the various thoracic regional techniques, fascial plane blocks are emerging as an effective alternative to conventional techniques such as paravertebral, epidural, or spinal blocks [17–19]. Fascial plane blocks involve the injection of a local anesthetic between the muscles through which the peripheral nerve travels [20]. For these techniques, the nerve itself is not targeted and the needle is not directed toward the neural axis; therefore, the risks of serious complications such as neural injury and neuraxial hematoma can be prevented or at least reduced. As most cardiac surgical patients receive heparin, avoiding neuraxial needling is especially appealing. The additional benefit that these blocks are relatively simple to perform has resulted in its widespread use.
In this article, the current evidence provided by randomized controlled trials (RCTs) on various ultrasound-guided fascial plane blocks for cardiac surgery are reviewed.
Method of the narrative review
We performed a literature search for articles assessing chest wall nerve blocks for cardiac surgery in PubMed, Embase, and Cochrane Library. The search was conducted from June 22, 2022 to June 26, 2022. In this review, the PICO (Populations of interest, Intervention, Comparators, and Outcomes) was as follows: (P) adults or pediatric patients undergoing cardiac surgery, (I) regional analgesia, (C) a comparative intervention such as no blockade or systemic analgesia, and (O) various clinical outcomes including pain score and analgesic consumption. The exclusion criteria were as follows: non-English language articles, non-RCTs, and duplicate or irrelevant articles. The search terms used included: (P) “Cardiac surgical procedures [Mesh] or “Heart surgery” or “Minimal invasive cardiac surgery” or “Anterior thoracotomy”; (I) limited to “PECS block,” or “Pectoral nerve block” or “Serratus plane block” or “Parasternal block” or “Transversus thoracis plane block” or “Erector spinae plane block” or “Retrolaminar block,” which are the most commonly performed thoracic fascial plane blocks.
We screened 1,223 records that were identified in the literature search. After removing duplicates, 1,026 distinct citations were retrieved, of which 991 (96.5%) were excluded during the initial screening phase. The primary reasons for exclusion were non-RCT designs and not meeting the inclusion criteria. After reviewing the full texts, 20 studies remained. Two additional cardiac surgery RCTs and three studies on subcutaneous-implantable cardioverter-defibrillator (S-ICD) implantation were included after a manual search was conducted. Consequently, 25 studies were included in this review; 22 for cardiac surgery (Table 1) and three for S-ICD implantation.
Nomenclature
Recently, the American Society of Regional Anesthesia and Pain Medicine (ASRA) and European Society of Regional Anaesthesia and Pain Therapy (ESRA) conducted an international study aimed at achieving consensus on the nomenclature of abdominal, paraspinal, and chest wall anesthetic techniques [21]. The names and anatomical definitions of various regional techniques were standardized by expert consensus. The suggested nomenclature was designed to make identifying the target injection point for each procedure easy. In this review, the descriptions will primarily be based on the new names. However, to show respect to the authors that first published studies of these techniques, the original nomenclatures are introduced at the beginning of each section. Table 2 summarizes the detailed injection points of the fascial plane blocks described in this article.
Fascial plane blocks for cardiac surgery
Parasternal intercostal plane block
The parasternal intercostal plane (PIP) block targets the anterior cutaneous branches of the intercostal nerves, which innervate the anteromedial chest wall. These nerves penetrate the intercostal muscles and pectoralis major muscle at each thoracic level. The block is divided into a superficial and deep PIP block according to the injection plane (beyond or below the internal intercostal muscle). Since the midline of the chest and abdominal wall slightly overlap on both sides, bilateral injection is required for complete coverage of the sternal area [22]. PIP blocks were performed for sternotomies in all the studies included in this section.
Superficial PIP block
The superficial PIP block was first introduced by Torre et al. in 2014 under the name “pecto-intercostal fascial plane block” [23]. Placing the ultrasound parasagittally on the lateral border of the sternum and using an in-plane technique allows for the fascial plane between the pectoralis major and internal intercostal muscles to be hydro-dissected and, once the needle is advanced along the dissection, allows for multi-level spread of the injectate (Fig. 1). Although this technique can be performed in the surgical field under direct vision at sternal closure, it is distinct from the ultrasound-guided approach since the spreading of the local anesthetic cannot be observed.
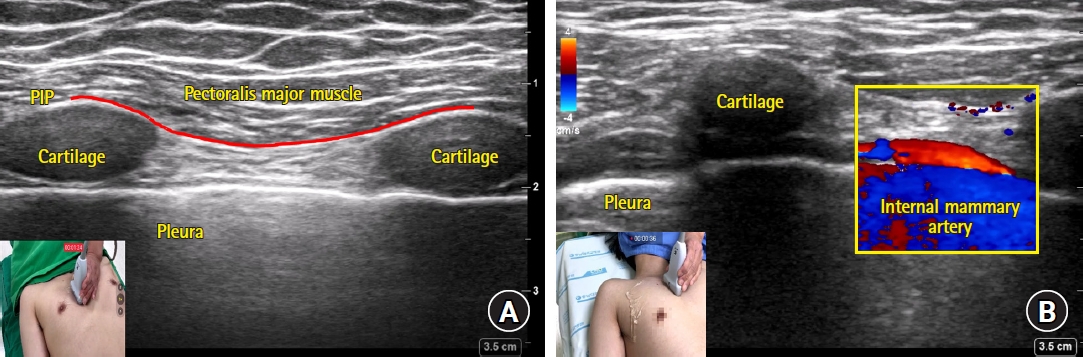
Sonoanatomy (A) and color doppler image of internal mammary artery (B) captured during parasagittal approach of superficial parasternal intercostal plane (PIP) block. (A) The fascial plane between the pectoralis major and internal intercostal muscles (PIP) is indicated as a red solid line. Cartilages were captured as echolucent structures. (B) The internal mammary artery is captured as a red-colored tubular structure on the long axis view.
Bloc et al. [5] assessed the effect of preoperative superficial PIP blocks on the intraoperative opioid requirement to maintain hemodynamic stability during sternotomy for coronary artery bypass graft (CABG) surgery. The maximal concentration of remifentanil was reduced in the block group (median 4.2 ng/ml vs 7.0 ng/ml in block and placebo groups, respectively) and significant reductions in postoperative proinflammatory cytokines were observed. Hamed et al. [24] evaluated the analgesic efficacy of the superficial PIP block after various open heart surgeries with a median sternotomy. The 24-hour cumulative morphine consumption was significantly reduced compared with the control group, although the effect size was small (–3.54 mg, 95% CI [−6.55, −0.53]). However, the quality of oxygenation (evaluated using PaO2 and the ratio of PaO2/inspired fraction of O2) in the postoperative period was improved in the block group. Khera et al. [25] also showed improved pain scores with the superficial PIP block compared to placebo, although the reduction in postoperative opioid consumption was not significant.
Deep PIP block
The anterior cutaneous nerve runs over the transversus thoracis muscle, traverses the intercostal and pectoralis major muscles near the sternal border, and enters the superficial plane. This allows for an injection to be performed on a more proximal and deeper plane. This technique was first described in 2015 as the “transversus thoracis plane block” [26]. Ultrasound scanning can be performed parallel to the lateral sternal border parasagittally or parallel to the intercostal space transversely (Fig. 1 and 2, respectively). The transversus thoracis muscle is a thin structure located just below the sternum and just above the pleura, making it difficult to be clearly distinguished on ultrasound imaging. Because the internal mammary artery (IMA) and vein run over this muscle, to avoid inadvertent puncture, these vessels should be visualized and used as a landmark (Fig. 1 and 2) [27]. Color-flow doppler is useful for probing the IMA, which is usually observed under translucent costal cartilage on an ultrasound image. The downward displacement of the pleura by the injectate can be used as an indication of appropriate ultrasound endpoint. Because the needle angle has to be stiffer in the parasagittal approach than in the transverse approach due to the narrow needle path (i.e., between the ribs), the transverse approach can be a little easier and safer for a deep PIP block. However, no study has compared the two approaches directly.
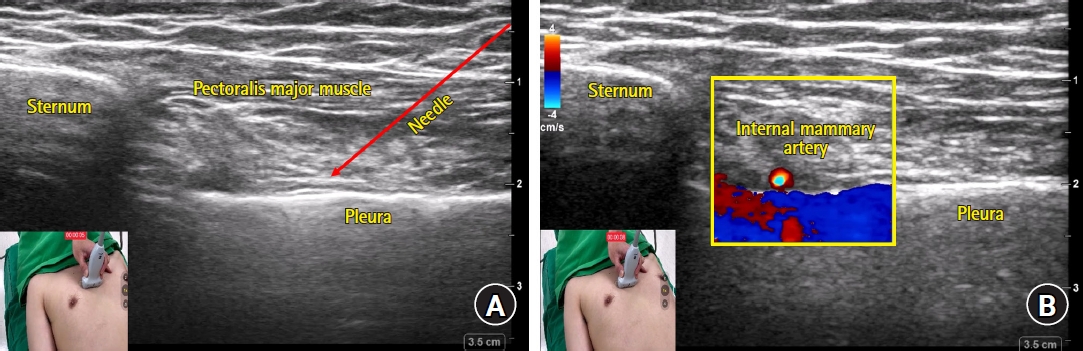
Sonoanatomy (A) and color doppler image of internal mammary artery (B) captured during transverse approach of deep parasternal intercostal plane (PIP) block. (A) The needle tip placement target is the fascial plane between the internal intercostal and transversus thoracis muscles. The transversus thoracis muscle is often difficult to clearly distinguish on an ultrasound image. The needle path of the transverse approach is indicated as a red solid line with an arrowhead. (B) The internal mammary artery is captured as a bright-colored round structure on the short axis image. It should be visualized and used as a landmark in order to avoid inadvertent punctures.
Fujii et al. [28] evaluated the safety and analgesic effect of the deep PIP block 12 h after cardiac surgery and found high rates of patient recruitment, adherence, and satisfaction. Aydin et al. [29] showed that a preoperative deep PIP block had a significant opioid sparing effect during the first 24 h postoperatively; however, compared with the placebo group, the improved pain score was only significant until 12 h post-operation. In a study conducted by Zhang et al. [30], the deep PIP block was found to provide not only an effective analgesia but also other positive clinical outcomes such as a shorter time to extubation, time to first bowel movement, and ICU and hospital stay.
The superficial PIP block is considered a safer technique than the deep PIP block as the former is performed further from vascular structures, the pleura, and the heart. However, since these are emerging techniques, reports about complications are scarce and further research is therefore warranted.
Use of PIP blocks in pediatric patients
In pediatric patients, a multi-level blockade can be achieved with a single injection of these PIP blocks, while adults require multi-level injections. In this section, recent RCTs of pediatric patients that show that PIP blocks could reduce intra- and/or postoperative opioid consumption and improve clinical outcomes will be discussed.
Abdelbaser et al. [31] revealed that the deep PIP block almost halved the opioid consumption in the first 24 h postoperatively and significantly lowered objective pain scores compared to the control group in pediatric cardiac surgery performed via a median sternotomy. Zhang et al. investigated deep [6] and superficial PIP blocks [32] through two RCTs. The block groups showed significantly lower pain scores in the first 24 h after extubation than the placebo group. But the pain scores were comparable between the groups at 48 h post-operation. In these studies, both blockades could also reduce the duration of mechanical ventilation and length of ICU stay, which are clinically meaningful outcomes.
Erector spinae plane block
The erector spinae plane (ESP) block has been used in various surgeries since it is both simple and safe to perform [33]. It was first introduced as a novel analgesic technique for thoracic neuropathic pain in a case report by Forero et al. in 2016 [34]. Usually, the clinician places the probe in the paraspinal area parasagittally and searches for a bony structure. Once the bony shape is identified, the clinician slides the probe in the medial and lateral directions to distinguish the round shape of the rib and the squared-off shape of the transverse process (Fig. 3A). While the pleura is clearly visible during rib scanning, this is not the case for transverse process scanning. The edge of the transverse process is the preferred target for needle placement, though slight advancement deeper off the edge may be needed to achieve the proper spreading of injectate into the plane between the erector spinae muscle and the transverse process [18].
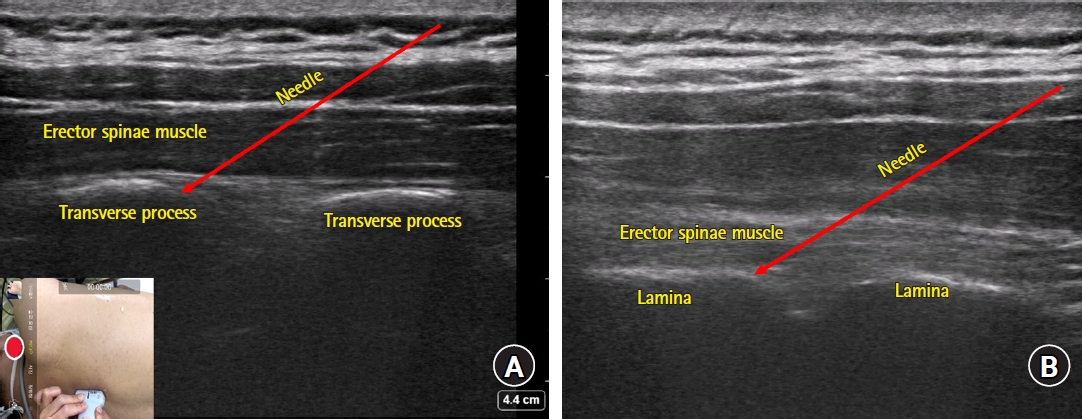
Sonoanatomy of erector spinae plane (ESP) (A) and retrolaminar (B) blocks. (A) Note the transverse process of the spine captured as bright squared-off bony structures underneath the erector spinae muscle. The edge of the transverse process is the preferred target for needle placement and slight advancement deeper off the edge may be needed to achieve proper spread of the injectate into the plane between the erector spinae muscle and the transverse process. (B) Note the flat structures (lamina) with small notches and the overlying erector spinae muscle. Using an in-plane technique, the needle can be introduced until the tip contacts with the lamina. Optimal needle positioning can be confirmed by observing the proper spread of injectate throughout the plane between the lamina and the erector spinae muscle.
The mechanism of the ESP block that mediates chest wall coverage (besides the back) appears to be the spread of the local anesthetic close to the paravertebral space where the dorsal and ventral rami of the spinal nerve diverge. Although there is plenty of evidence showing the analgesic effect of the ESP block in various types of surgery including breast, thoracic, and abdominal surgery, its use in cardiac surgery is still limited [35–39]. The ESP block has some advantages in terms of safety and simplicity over the thoracic paravertebral or epidural blocks, which are associated with risks such as hematomas, neural injury, or pneumothorax and are technically challenging.
In their study assessing the efficacy of a single-shot ESP block in adult cardiac surgery, Athar et al. [8] found superior analgesia with a decrease in opioid consumption of 64.5% and a greater reduction in the duration of mechanical ventilation and the sedation score at 6 h post-extubation compared with the sham block. Krishna et al. [40] compared the single-shot ESP block with a conventional analgesic regimen in cardiac surgeries with cardiopulmonary bypass, which included intravenous paracetamol and tramadol. Interestingly, the analgesic effect of the ESP block was almost perfect in the immediate postoperative period, as shown by a median NRS score of 0 until the sixth hour post-extubation.
The studies described above evaluated the efficacy of a single-shot ESP block. As the gradual anterior spread of the block to the thoracic paravertebral space is considered a determinant factor for consistent coverage of the anterolateral chest wall [41], a continuous block may provide a greater analgesic effect by gradual diffusion of local anesthetic over a prolonged period of time. Wasfy et al. [42] evaluated a continuous bilateral ESP block in CABG surgery and found a reduction in the postoperative opioid consumption and pain scores in the block group until 48 h post-extubation. Additionally, they found improved peak inspiratory flow and a reduction in the duration of mechanical ventilation and ICU stay.
Recently, the pathways through which the injectate spreads to the paravertebral space has been further elaborated for the ESP block [43–45]. The superior costotransverse ligament (SCTL) incompletely forms the posterior wall of the thoracic paravertebral space and through the slits (including the costotransverse foramen), the retro-SCTL space broadly communicates with the paravertebral space. Thus, the so-called intertransverse process (ITP) blocks that involve administering the injection a little closer to the communicating channels (between the retro-SCTL and paravertebral spaces) than the ESP block have been introduced under various names [46]. Future research evaluating the usefulness of ITP blocks in cardiac surgery is needed.
Use of ESP block in pediatric patients
In 2017, Chin et al. [47] reported a successful perioperative pain management using ESP block in a pediatric patient who underwent oncological thoracic surgery. Since then, the ESP block has been applied in a wide range of pediatric clinical scenarios [48].
Kaushal et al. [7] evaluated the efficacy of the ESP block in pediatric patients with acyanotic congenital heart disease undergoing cardiac surgery via a midline sternotomy. Pain scores were significantly reduced by the blockade until 10 h post-extubation. Additionally, rescue opioid consumption and the duration of the ICU stay were reduced with lower sedation scores in the ESP block group. Conversely, Karacaer et al. [49] noted no significant differences between the ESP block and control groups in terms of the pain score, sedation score, extubation time, and length of ICU stay. Gado [50] assessed the efficacy and safety of bilateral ESP blocks in pediatric patients undergoing cardiac surgeries through a median sternotomy. Perioperative opioid consumption was reduced in the ESP block group compared with the control group with comparable postoperative complications such as vomiting, itching, and respiratory depression. Macaire et al. [51] performed a bilateral continuous ESP block in pediatric cardiac surgery performed via median sternotomy. Significantly less total morphine consumption during the first 48 h postoperatively and improved pain scores were noted in the block group compared with the control group. A protocol consisting of programmed intermittent bolus injections through bilateral catheters (alternatively) was used to avoid systemic toxicity associated with local anesthetics in the study. The plasma ropivacaine concentrations at 1 and 48 h after the initiation of the blockade were below known safe levels.
Retrolaminar block
The retrolaminar block first appeared in 2013 in a case report on pain management of a patient with multiple rib fractures [52]. Similar to the ESP block, the retrolaminar block is performed in the parasagittal plane. Sliding the probe from the lateral to medial direction, the rib, transverse process, and lamina can be distinguished by their distinctive bony contours which are round, rectangular, and flat structures with small notches, respectively (Fig. 3B). Using an in-plane technique, the needle can be introduced until the tip contacts with the flat structure (i.e., lamina). Optimal needle positioning can be confirmed by observing the proper spreading of injectate throughout the plane between the lamina and erector spinae muscle.
Although this blockade is different from the ESP block in that the injection point is the plane between the erector spinae muscles and the lamina [21], the mechanism of this block is similar to that of the ESP block [53]. Currently, the analgesic efficacy of the so-called “paravertebral by proxy” techniques (ESP, retrolaminar, and ITP blocks) is mainly explained by how much the injectate spreads anteriorly to the paravertebral space [54].
Only one recent RCT [55] to date has reported on an ultrasound-guided bilateral thoracic retrolaminar block in pediatric open cardiac surgery. Perioperative fentanyl consumption was significantly lower in the block group compared with the control group. Additionally, the block enabled early extubation and a shortened ICU stay.
Interpectoral plane, pectoserratus plane, and serratus anterior plane blocks
PECS I (currently the interpectoral plane [IPP] block), PECS II (currently the IPP combined with the pectoserratus plane [PSP] block), and the serratus anterior plane (SAP) blocks were first introduced by Blanco et al. [56–58]. The IPP block targets the medial and lateral pectoral nerves and is performed by injecting local anesthetic within the fascial plane between the pectoralis major and minor muscles [56]. The PSP block targets the lateral cutaneous nerve within the fascial plane between the pectoralis minor and serratus muscles (Fig. 4A). Since the PECS II block actually consists of two blocks (the IPP and PSP), it is sometimes erroneously described as the PECS I + PECS II block [57]. Thus, in this review, the PECS II block is described as the IPP-PSP block to avoid confusion. The SAP block was introduced as a modification of the PECS blocks, involving an injection that is more lateral and posterior in order to provide analgesia for most of the hemithorax [58]. It is further divided into the superficial and deep SAP blocks, depending on whether the injection is performed at the plane above or below the serratus anterior muscle (Fig. 4B). Therefore, the target plane for the PSP and superficial SAP blocks is actually the same, though the injection is either performed in the area under the pectoralis minor muscle (PSP) or not (superficial SAP).

Sonoanatomy of interpectoral plane (IPP) and pectoserratus plane (PSP) blocks (A), and deep and superficial serratus anterior plane (SAP) blocks (B). (A) The fascial planes between pectoralis major and minor muscles (IPP) and pectoralis minor and serratus anterior muscles (PSP) are indicated as solid red lines. Note that the probe is placed at the right upper quadrant of the chest wall (over the pectoralis muscles). (B) The planes superficial (superficial SAP) or deep (deep SAP) to the serratus anterior muscle are indicated as solid red lines. Note that the probe is placed at the lateral chest wall.
Kaushal et al. [59] compared the efficacy of the ultrasound-guided deep SAP block, IPP-PSP block, and intercostal nerve block for the management of post-thoracotomy pain in pediatric cardiac surgery. These blocks showed comparable efficacy in terms of pain scores in the early postoperative period (1 to 4 h), but a more prolonged analgesic effect was found in the SAP and PSP groups.
The mechanism by which the IPP or PSP block mediates the analgesic effect after sternotomy is unclear. Although these blocks are not expected to cover the anterior cutaneous branches of the intercostal nerves, several studies have reported effective analgesia of the PSP in pediatric cardiac surgery involving median sternotomy [60,61]. In pediatric patients, while the local anesthetic injected in the anterolateral chest wall may spread to the anteromedial side given the relatively small size of the chest wall, direct evidence is currently lacking. It can hardly be expected, however, that the analgesic effect of these blocks would be sufficient for adult patients post-sternotomy [62].
Kamal et al. [60] compared the analgesic effect of the bilateral IPP-PSP block to conventional intravenous analgesia (control) for post-sternotomy pain after pediatric cardiac surgery. The block group reported lower pain scores and postoperative opioid requirements than the control. Furthermore, emergence agitation and the duration of the ICU stay were also lower in the study group. Kumar et al. [61] performed a bilateral IPP-PSP block in cardiac surgeries performed via a midline sternotomy. The block group required a shorter duration of ventilator support in comparison to the parenteral analgesia group, and the analgesic effects and inspiratory function also improved.
A study conducted by Gawęda et al. [63] provides interesting insight into the distinct mechanism of the IPP-PSP block. In that study, patients undergoing mitral/tricuspid valve repair via mini-thoracotomy were randomized into either the ESP or ESP with IPP-PSP block groups. The ESP with IPP-PSP block group showed better analgesic outcomes and patient satisfaction. In theory, the most distinctive nerves that could be blocked by the IPP-PSP block that are not covered by the ESP block are the pectoral nerves. As the pectoral nerves are motor nerves, the analgesic effect expected from the blockade of these nerves would mainly be due to a reduction in pectoral muscle spasm. The additional analgesic effect provided by the IPP-PSP block in the study could be partially explained by this mechanism.
The SAP block is performed more posterolaterally on the chest wall than the IPP or PSP blocks [58]. Sufficient hydro-dissection of the fascial plane provides a multi-level blockade of the lateral cutaneous branches of the intercostal nerves. This technique can be performed in a supine or lateral decubitus position and thus is flexible according to the planned surgical positioning (e.g., supine position for MIDCAB or lateral decubitus position for lung surgery) (Fig. 4B). Although significant analgesic effects have been shown for thoracoscopic surgery, the SAP block appears to have less of an analgesic effect than the paravertebral or ESP blocks [38]. In the only RCT using the SAP block in cardiac surgery to date, Gautam et al. [64] evaluated the role of a continuous deep SAP block for postoperative pain relief in patients undergoing MIDCAB surgery via left anterior thoracotomy. Although reduced pain scores and postoperative opioid consumption were reported for the SAP block group, fentanyl was included in the infusate for the continuous blockade (1 μg/ml, infused at 8 ml/h after 20 ml of bolus dose), and thus the efficacy of the SAP block itself in the study is uncertain.
Fascial plane blocks for subcutaneous-implantable cardioverter-defibrillator implantation
A proper regional analgesia for S-ICD implantation should cover two areas of the chest wall, one for a pocket creation between the serratus anterior and latissimus dorsi muscles and another for parasternal tunneling of the lead. A well-established guideline addressing regional techniques for S-ICD implantation is still lacking and only a recommendation led by U.S. physicians published in 2018 exists to date [65]. A few recent studies on the SAP and PIP blocks, however, have been conducted.
Shariat et al. [66] investigated the efficacy of a deep PIP block combined with a superficial SAP block for S-ICD implantation. Compared with the wound infiltration group, intraoperative fentanyl requirements were significantly lower in the block group. However, as the administration of intraoperative fentanyl was based on the subjective discretion of a non-blinded anesthesiologist, the validity of the positive result of the study is uncertain. A larger, double-blinded RCT [67] has been conducted that compares the analgesic efficacy of the regional techniques consisting of the ultrasound-guided deep PIP block and deep SAP block versus local infiltration in 80 S-ICD placements. The pain scores assessed by the Critical-Care Pain Observation Tool during the procedure were significantly lower in the block group compared with the local infiltration group. The authors conducted another similar study using the same techniques in pediatric patients [68]. The block group showed favorable analgesic outcomes and shorter extubation time and length of PACU stay compared with the control group (sham block).
Conclusions
Evidence suggests that fascial plane blocks can provide effective analgesia for cardiac surgery. Some studies additionally provide evidence of improved postoperative pulmonary mechanics and reduced length of ICU or hospital stays. However, the followings should be considered when interpreting the results of the previous studies and applying them to clinical practice:
1. The coverage of the blockade should correspond to the target surgical site. Specifically, sternal coverage of the IPP and PSP block is not guaranteed in adult patients.
2. The consistency of the blockade may depend on several factors. The efficacy of paravertebral by proxies can be influenced by the degree of paravertebral spread of the injectate. Additionally, a lack of delicate control during needle tip placement can impair proper spreading of the injectate into the target plane and thus hinder block consistency.
3. The somatic blockade does not guarantee complete analgesia for cardiac surgery; therefore, it may be more effective in combination for multimodal analgesia.
4. Although the included studies in this review showed positive outcomes for various cardiac surgeries, functional or long term clinical outcomes are limited [69].
A comprehensive conclusion regarding the efficacy of ultrasound-guided fascial plane blocks in cardiac surgery cannot be made since more studies comparing various techniques are needed. Nevertheless, most fascial plane blocks for cardiac surgery show effective analgesia and low procedure-related risks. Therefore, the application of these emerging ultrasound-guided fascial plane block techniques in cardiac surgery is worthy of consideration.
Notes
Funding
This work was supported by the research fund of National Research Foundation of Korea (NRF-2022R1C1C1007982) and Chungnam National University.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Boohwi Hong (Funding acquisition; Investigation; Project administration; Writing – original draft; Writing – review & editing)
Chahyun Oh (Visualization; Writing – original draft; Writing – review & editing)
Yumin Jo (Writing – original draft; Writing – review & editing)
Soomin Lee (Writing – original draft; Writing – review & editing)
Seyeon Park (Data curation; Investigation; Writing – review & editing)
Yoon-Hee Kim (Project administration; Supervision; Writing – review & editing)


