Erector spinae plane block in children: a narrative review
Article information
Abstract
The erector spinae plane block (ESPB) is a novel technique used in both adult and pediatric patients. Its use in children has mostly been described in terms of perioperative pain management for various types of surgery. After its introduction, anesthesiologists began using ESPBs in various surgical settings. As adequate analgesia along with a low complication rate were reported, interest in this technique dramatically increased. Many studies in adults and children, including randomized controlled trials, have been published, resulting in the emergence of different clinical indications, with various technical and pharmacological approaches currently evident in the literature. This narrative review aims to analyze the current evidence in order to guide practitioners towards a more homogeneous approach to ESPBs in children, with a major focus on clinical applications. The ESPB is an efficient, safe, and relatively easy technique to administer. It can be applied in a wide range of surgeries, includes thoracic, abdominal, hip, and femur surgery. Its usefulness is evident in the context of enhanced recovery after surgery protocols and multimodal analgesia. Single-shot, intermittent bolus, and continuous infusion techniques have been described, and non-inferiority has been observed when compared with other locoregional techniques. Even though both the efficacy and safety of the procedure are widely accepted, current evidence is predominantly based on case reports, with very few well-designed observational studies. Consequently, the level of evidence is still poor, and more well-designed double-blind, randomized, placebo-controlled trials are needed to refine the procedure for different clinical applications in the pediatric population.
Introduction
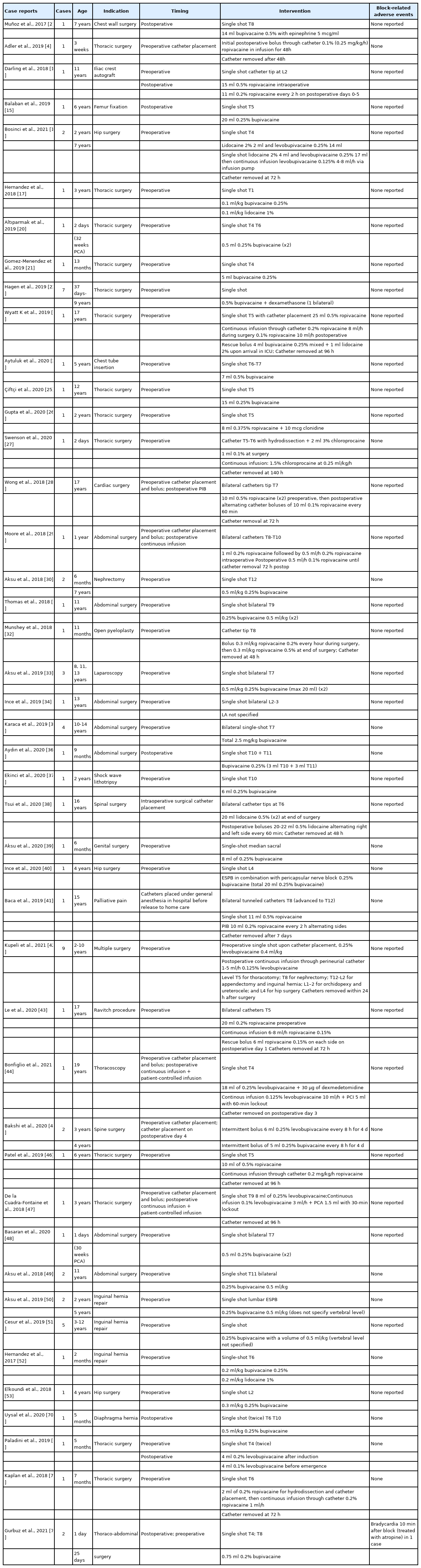
The erector spinae plane block (ESPB), which was first described by Forero in 2016 [1] for the treatment of thoracic neuropathic pain, was applied in the pediatric population for postoperative pain management as early as 2017 [2]. Subsequent interest in this technique has rapidly expanded and expertise has increased, with applications not only for the management of perioperative analgesia but also for non-surgical pain management in the pediatric population (Table 1).
Despite more extensive series and observational evidence in the current literature, very few rigorous, well-designed trials have been conducted (Table 2). While these include a few randomized controlled studies, the protocols and indications used have been highly variable. The efficacy and safety of ESPBs in perioperative pain management have been explored in many case reports and observational studies compared with other loco-regional procedures (Tables 1 and 2). However, the position, timing, and pharmacological approach are highly variable across operators and no standardized protocols are available.
The level of evidence is still limited, and a general consensus on these aspects is lacking. The purpose of this narrative review is therefore to provide an overview of the state of ESPBs in children, highlighting critical aspects and future perspectives to guide practitioners towards a more homogeneous approach.
Clinical indications
ESPBs have been proposed for a wide variety of potential applications, especially as part of a multimodal perioperative analgesia regimen to promote enhanced recovery after surgery (ERAS) in numerous kinds of procedures, ranging from chest and abdominal to inguinal and lower limb surgeries. In fact, analgesia can be attained in a broad range of anatomical areas depending on the vertebral level of local anesthetic (LA) injection, with extensive craniocaudal spread providing anesthesia coverage to multiple dermatomes [3]. Consequently, this technique allows for the operator to achieve analgesia in the desired region with an injection that is remote from the surgical incision area [2].
While ESPBs could be synergistic with other locoregional techniques, to date, it has mostly been reported as an alternative to other approaches. For example, the ESPB has been used as an alternative to the thoracic epidural, a classic technique for cardiothoracic surgeries involving a midline sternotomy or thoracotomy, as it is considered safer because it is injected farther from important structures, such as the spinal cord, pleura, and vascular structures [4]. Bilateral ESPBs at the T3-T4 level have been shown to provide improved postoperative analgesia compared to no block in children undergoing cardiac surgeries involving a midline sternotomy [5,6].
Several reports have described the use of ESPBs for perioperative pain management during lower body surgeries. The non-inferiority of the ESPB to the quadratus lumborum block in pediatric lower abdominal surgeries has been shown [7]. Bilateral ESPBs can provide superior intraoperative and postoperative analgesia compared to sham blocks for splenectomies involving a midline incision [8] and compared to no block for lower abdominal surgeries [9]. ESPBs have also been shown to provide more effective and longer-lasting postoperative analgesia than ilioinguinal/iliohypogastric blocks in unilateral inguinal hernia repairs [10].
Despite the fact that caudal epidurals are one of the most extensively used regional blocks in children undergoing hip and lower abdominal surgeries [11,12], no studies have compared caudal epidurals to ESPBs in pediatric patients. Additionally, no comparisons between ESPBs and psoas compartment blocks in hip surgery have been conducted, even though the effectiveness of both techniques have been confirmed [13–16].
A reduction in the use of intraoperative and postoperative analgesics for opioid-sparing general anesthesia has also been demonstrated with ESPBs [5,6,8]. Anecdotal evidence in the form of case studies and small case series have been reported for numerous applications of the ESPB as part of a multimodal opioid-sparing analgesia regimen, including for thoracotomy, thoracoscopic surgery, thoracic wall surgery [2,3,4,18–27], sternotomy [28], abdominal surgery [29–37] spinal surgery [38], genital surgery [39] and hip joint and proximal femur surgery [14–16,40].
Outside the operating room, ESPBs have been successfully employed for pain control in pediatric oncological palliative care [41].
Beyond the use of ESPBs as a routine technique in the pediatric anesthesiologist’s arsenal, it has also been applied in the management of particularly complex cases. Due to the craniocaudal spread of ESPBs, it can be injected at a remote point from the vertebral level of the incision site [42], and thus may be used when there is an incision site infection or as a valid alternative to neuraxial anesthesia in the case of spinal deformities, previous spinal surgery, or neuraxial spread of neoplastic disease [43–45]. ESPB catheters have also been used successfully when epidural and paravertebral catheters are not possible due to coagulopathy [46]. The high safety profile of the ESPB in terms of the hemodynamic impact has led to its consideration by clinicians who are reluctant to use epidural anesthesia in patients with heart defects [47]. Additionally, during the perioperative period of an emergency laparotomy, analgesia has been provided by ESPBs even in very low birth weight premature infants, despite their small size [48].
Anatomy, technique, and diffusion of the anesthetic solution
Accurate knowledge of pediatric anatomy is essential for performing an ESPB and achieving an adequate sensory blockade. Anatomical differences between adult and pediatric patients, such as the muscles, fascia, and connective tissues under the skin, which are usually thinner and less rigid in pediatric patients, must be taken into account. Therefore, neonatal/pediatric probe, shorter needle, and lower drug volume should be considered.
Regardless of the vertebral level, the target of this fascial plane block is the erector spinae fasciae plane. This is a virtual space located under the erector spinae muscles that communicates with the paravertebral space where the dorsal rami of the spinal cord is located (Fig. 1).

(A) The ultrasound probe is placed along the midline of the spine and moved laterally to visualize the transverse process. (B) The needle advances to the tip of the transverse process, and LA is injected to dissect the plane deep to the erector spinae muscle.
The erector spinae muscles constitute the intermediate layer of the deep muscles of the vertebral column, arising from the posterior part of the iliac crest, sacrum, and lumbar spinous processes. It encompasses the spinalis, longissimus dorsi, and iliocostalis muscles. These muscles are located posterolaterally to the vertebral column, lying between the vertebral spinous processes medially and angles of the ribs laterally.
During sonography, the probe should initially be placed at the midline of the spine with a transverse orientation to visualize the spinous processes. Moving laterally, the transverse processes can then be located. Maintaining the probe in a transverse orientation, an out-of-plane approach can be used, with the needle placed in a craniocaudal or caudocranial direction. Otherwise, after a 90° rotation of the probe, an in-plane approach is also possible.
The structures are visualized from superficial to deep as follows: the trapezius, rhomboid, and erector spinae muscles and the transverse process of the respective vertebra.
The needle must be advanced to the tip of the transverse process, after which the LA can be injected to hydrodissect the plane deep to the erector spinae muscle to verify the proper injectate location before injecting the residual volume. Alternatively, if the patient’s weight allows for only very small volumes of LA, normal saline may be used for this initial hydrodissection to spare the LA allowance.
The distance between the skin and tip of the transverse process is very small in pediatric patients and can vary considerably according to age and body mass index. Therefore, small-sized needle devices and a stable position are required to perform the block.
The above mentioned approach, which is mostly conducted in the prone position in children, is similar to that used in the first reports of ESPBs conducted on two adult patients in the sitting position [1]. As this technique has been increasingly applied in the pediatric population and refined for the particular needs of these patients, several other approaches have been developed. In 2018, an ESPB administered with the patient in the lateral decubitus position was described by placing the ultrasound probe transversely to obtain a midline view of the spinous and transverse processes of the vertebral and erector spinae muscles, using an out-of-plane technique [49].
The Aksu approach for lumbar ESPBs has also been described in pediatric patients, in which an in-plane technique in the lateral decubitus position is applied, thus eliminating the need to turn the anesthetized patient prone and then back to a supine position for surgery [50]. The major disadvantage of this approach is the inability to visualize the craniocaudal spread of the LA, which is only possible when the probe is turned to the sagittal position after the block is performed [51].
Regardless of the technical approach, LAs injected into the erector spinae fascial plane are meant to spread through the paravertebral space, not only at the level of the injection site but also cranially and caudally to reach distant dermatomes. However, the exact diffusion pathway remains controversial. The dorsal rami emerges from the paravertebral space and moves through the inter-transverse connective tissue complex. The ventral rami continues from the paravertebral to the intercostal space, becoming the intercostal nerves. The involvement of the ventral rami is contested since no solid evidence is available on the actual route of spread of the injected drugs.
Different methods have been adopted to study the spread of LAs; however, most are described in adult patients whose tissues are much more rigid and stiffer than those of children. Sonography, while clearly a limited technique, is useful for studying the cephalocaudal distribution of the injectate and is feasible in the pediatric population. Munoz et al. [2] observed the spread of LA from T5 to T11 after an 8-ml injection of solution was performed at T8 in a 7-year-old patient. Additionally, the distribution of a 3.2-ml solution from T1 to T9 was documented via ultrasound in a 3-year-old patient after an ESPB was performed at T1 [17]. In another case report, the same author visualized the spread of 1 ml of solution between T4 and L1 following an ESPB performed at T6 in a 2-month-old infant [52]. A wide distribution was also observed between L1 and L4 following the administration of 4.5 ml of solution at L2 in a 4-year-old patient [53].
A cadaveric study that analyzed the spread of a methylene blue dye solution in two embalmed preterm stillborn neonates weighing 1.6 and 0.6 kg was also conducted. The first cadaver received a unilateral 0.5-ml injection of solution at the T5 level, and superficial cephalocaudal diffusion from T2 to T12 was observed, with deeper staining of the ventral and dorsal roots and ganglia between T3 and T6. In the second cadaver, in which a 0.2-ml injection was performed at the T8 level, superficial staining spread from T7 to L1 and dorsal and ventral roots/ganglions were involved from T7 to T11. In both cases, the paravertebral and epidural spaces as well as the dura mater surrounding the spinal cord were stained [54].
CT scanning with multi-slice and three-dimensional (3D) technology has been used to assess the spread of iodinated contrast dye in a fresh unembalmed preterm neonatal cadaver weighing 2.7 kg. The first injection (2.3 ml) was performed at the T8 level on the right side and a second injection (2.3 ml) on the opposite side was performed at the T10 level. 3D reconstruction revealed diffusion of the dye from T6 to T9 on the right side and from T9 to T11/T12 on the left side. Contrast dye was seen in the paravertebral space but not in the epidural space, spreading over the costotransverse ligament and reaching the intercostal space. The lack of spread to the epidural space could be explained by in vivo factors, such as intrathoracic pressure changes and the absence of muscle tone and tissue tension. The study suggested a volume of 0.3–0.4 ml per dermatome, with involvement of 3 to 4 dermatomes with the ESPB [55].
The paucity of data available regarding injectate spread in children requires that adult studies be referenced. However, considerable differences in diffusion patterns in adult and pediatric patients must be recognized in relation to multiple factors, such as the developmental formation of the vertebral curvature, more elastic pediatric spine, and less dense ligaments and cartilaginous laminae [54]. Drug distribution in the adult population has been observed in MRI and cadaveric studies, which suggest different possibilities for lateral and anterior diffusion of LAs. Beyond anatomy, the vertebral level [56] and drug volume [57] are also relevant factors in the spread of injected LA. Analyses of cadaveric samples have revealed anterior and posterior diffusion of the injectate with different percentages at different vertebral levels, with inconclusive results. Paravertebral, intercostal, and epidural spread have been described, but these findings are not consistent among the available studies [56–68]. Given these variabilities in adult MRI and cadaveric studies, the results are inconclusive and presumably related to the site of injection, volume of solution, and physical characteristics of the tissues.
Although this technique is clearly effective, given its many successful clinical applications, evidence regarding the precise diffusion of the injected solution in children is not entirely clear, with only two studies on neonatal cadavers [54,55] and several case series reporting data from in vivo sonographic imaging.
Choice and dosage of local anesthetics
The pharmacological approaches described in the current literature are highly inconsistent, as the procedure has multiple applications and the specific pharmacological approach associated with the variety of clinical contexts is variable.
Bupivacaine, ropivacaine, and levobupivacaine at different concentrations and volumes have been most commonly used for ESPBs in pediatric patients, with no significant differences in postoperative pain management between them.
In the first documented application of the ESPB in children in the literature, a single shot injection of 14 ml of 0.5% bupivacaine performed at T8 was administered to a 7-year-old boy undergoing surgery for the treatment of a tumor of the eleventh rib [2].
Most of the pediatric ESPBs currently described in the literature are performed with 0.25% bupivacaine, with volumes ranging from 0.3 to 0.6 ml/kg [19,20,25,30,31,33,36,69,70].
A 1 : 1 solution of 0.25% bupivacaine and 1% lidocaine with a total volume of 0.2 ml/kg was administered via ESPB as a single shot injection to a 3-year-old girl weighing 16 kg undergoing surgical resection of dorsal lipoma. The patient was discharged 4 h after surgery with full pain control [17]. The same solution at a dosage of 0.4 ml/kg was administered as a single shot ESPB in a 2-month-old infant before inguinal hernia repair [53].
The use of levobupivacaine 0.2% (4 ml) for thoracic surgery in a 5-month-old female was also reported [71] to enhance recovery after surgery.
The first continuous ESPB in the literature was administered to a 3-year-old boy, who received an 8-ml initial injection of 0.25% levobupivacaine through a catheter placed at the T9 level at the end of a thoracotomy. Two hours later, a patient-controlled analgesia pump with 0.1% levobupivacaine (3 ml/h continuous infusion) was started, with a standing order of 1.5-ml rescue boluses at 30-min lockout intervals. On the fourth postoperative day, the infusion was stopped. Only two 1.5-ml rescue boluses were administered and no other medications were required to control pain [47].
A 7-month-old infant received 0.2% ropivacaine through a catheter placed at the T6 level (1 ml/h) prior to an upper lobectomy for a congenital pulmonary airway malformation. The catheter was removed on the third postoperative day, and pain scores showed adequate analgesia [72].
A retrospective review of a single center on various surgeries described the efficacy of ESPBs with 0.5% ropivacaine in children, with an initial loading dose of 0.4 ml/kg followed by intermittent boluses of 0.2%–0.3% at 0.3 ml/kg administered hourly [73].
Pharmacokinetic variability must be taken into account for all fascial plane blocks. In contrast to peripheral nerve blocks, where anesthetics are precisely deposited around a specific nerve, a consistent level of intensity of the sensory blockade cannot be expected for fascial plane blocks. Moreover, differences in tissue laxity in pediatric patients could contribute to increased variability. Consequently, it is difficult to replicate the same sensory block in different children, even when administered by the same practitioner.
More studies are thus necessary to guide the type, dosage, and duration of LA and adjuvant administrations to create specific protocols for the various clinical applications of pediatric ESPBs.
Safety profile and adverse events
Regional anesthesia is generally considered safe in the pediatric population, although caution must be exercised, especially when applying these techniques to infants [74]. Furthermore, these techniques can be safely utilized under general anesthesia [75]. In particular, ESPBs appear to be exceptionally safe, as the injection site is very superficial and ultrasound guidance allows for visualization of vital structures such as the neuraxis, pleura, and vascular structures as the needle is inserted. Additionally, it is widely accepted that ESPBs can be conducted safely in patients with coagulopathy [46]. While the standard contraindications and possible complications of any peripheral block, including LA systemic toxicity (LAST), allergic reactions, or motor block, may occur with ESPBs, the available literature documents a promising safety profile in children, with most studies reporting no adverse events or complications such as epidural hematoma, which may occur with neuraxial blocks.
Some minor adverse events such as catheter occlusion, displacement, and unintentional removal have been reported [6,73]. Rare cases of bradycardia or possible LAST have also been reported, but quickly reversed [76,77]. While injection site infections may be a contraindication to peripheral nerve blocks, the possibility of injecting LAs at a site distant from the target in a fascial plane block may allow operators to safely implement ESPBs even in cases of surgical site infection. One other potential complication of ESPBs is a pneumothorax [78]; however, a literature search yielded no documented episodes, and experienced operators hold that ultrasound guidance, fine needle skills, and preventative techniques can minimize this risk [49].
Conclusions
Despite significant interest in ESPBs in the pediatric anesthesia community due to its versatility, low learning curve and safety profile, the available evidence is still anecdotal and non-homogeneous with few rigorous trials, yielding low-quality evidence and no clear protocol to follow. Taken together, the available data suggest that ESPBs may be a valid technique to improve intra- and postoperative pain control and reduce opioid use in pediatric thoracic, abdominal, inguinal, hip, and femur surgeries. Additionally, multiple authors have considered this procedure a valid alternative to other loco-regional techniques and epidural anesthesia. The choice of LA is quite variable among practitioners, with reports of single shot or continuous infusions for different surgeries, and the distribution of injected solutions remains controversial. Hence, more well-designed randomized controlled trials are needed to clarify specific approaches for performing ESPBs for different clinical procedures in the pediatric population.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Monica Lucente (Conceptualization; Data curation; Writing – original draft)
Giulia Ragonesi (Data curation; Funding acquisition; Writing – original draft; Writing – review & editing)
Marco Sanguigni (Data curation; Software; Writing – original draft)
Fabio Sbaraglia (Conceptualization; Methodology; Supervision; Writing – review & editing)
Alessandro Vergari (Resources; Validation; Visualization; Writing – review & editing)
Rosa Lamacchia (Resources; Writing – review & editing)
Demetrio Del Prete (Data curation; Writing – review & editing)
Marco Rossi (Conceptualization; Writing – review & editing)