Feasibility and efficacy of erector spinae plane block versus transversus abdominis plane block in laparoscopic bariatric surgery: a randomized comparative trial
Article information
Abstract
Background
Overweight and obesity are growing public health concerns worldwide. Bariatric surgery is a modality of weight reduction; however, postoperative pain can increase the length of hospital stay, with all the associated consequences. While regional anesthesia is an available option, the feasibility of performing abdominal wall blocks on patients with obesity is questionable.
Methods
Sixty adult patients with a body mass index of 40–50 kg/m2 undergoing laparoscopic bariatric surgery were randomly assigned to receive either an ultrasound-guided transversus abdominis plane (TAP) or erector spinae plane (ESP) block. The primary outcome was the analgesic effect in the first 24 h postoperatively, assessed using the mean visual analog scale (VAS) score. Secondary outcomes were the time required for a successful block, incidence of complications, time to first rescue analgesia, time to flatus or stool passage, and total opioid consumption.
Results
The mean VAS score during the first 24 h was higher with the TAP block than with the ESP block (2.78 ± 0.34 vs. 2.32 ± 0.12, P < 0.001). Additionally, the time to first rescue analgesia was greater with the ESP block (P = 0.001) and the time required for a successful block was higher with the TAP block (P = 0.001). However, the incidence of complications, total opioid consumption, and other secondary outcomes was similar between the groups.
Conclusions
Compared with the TAP block, the bilateral ESP block is a more feasible and effective method for intra- and postoperative analgesia in patients undergoing laparoscopic bariatric surgery.
Introduction
Obesity and overweight are growing public health concerns worldwide [1]. Since bariatric surgery can be an efficient method for managing obesity, the number of bariatric surgeries performed worldwide is increasing, resulting in an increase in number of patients suffering from the accompanying postoperative consequences [2].
Postoperative analgesia presents various challenges in vulnerable patient groups suffering from obesity. With the high potential risk of respiratory depression and postoperative pulmonary complications associated with opioid use, such as atelectasis and pneumonia, the availability of other pain management modalities is essential [3].
Transversus abdominis plane (TAP) blocks could provide a modality for postoperative pain management as part of a multimodal pain control regimen. The subcostal approach targets the innervation of the upper abdominal wall and provides analgesia for trocar site insertion in laparoscopic surgeries [4]. However, studies on the TAP block with a range of surgical procedures have demonstrated that it may not always reliably capture the T7 and T8 dermatomes. Sensory blocks at these dermatomes are necessary to achieve analgesic satisfaction in cases of laparoscopic abdominal surgery. Therefore, the feasibility of the TAP block in patients with obesity and a lack of visceral pain control may be low [5].
The ultrasound-guided erector spinae plane (ESP) block is a novel interfascial block that provides postoperative analgesia to the abdominal wall [6]. The ESP block could offer wider abdominal wall analgesic coverage along with visceral pain control [7], which would be an advantage over the TAP block. However, the main concern with the ESP block is feasibility together with the potency of the block in challenging populations, such as patients suffering from obesity.
Both blocks could provide effective postoperative analgesia. In this study, we aimed to compare the postoperative analgesic effect and feasibility of both the TAP and ESP blocks in patients with obesity undergoing bariatric surgery.
Materials and Methods
Methods
This was a prospective, double-blind, randomized clinical trial. The institutional Research Ethics Committee of Cairo University El-Kasr Alainy Hospital approved this study (IRB no. MD-250-2020). The trial was registered on clinicaltrials.gov (Ref no. NCT 04417179) and was conducted from January 2021 to February 2022 in accordance with the Helsinki Declaration-2013. All patients who were screened and met the eligibility criteria were invited to participate in the trial, and all enrolled patients provided written informed consent. Consent was requested from patients upon arrival to the operating suite for surgery or on the ward if they were admitted the night before surgery.
The inclusion criteria were as follows: patients of any gender aged 18–60 years with American Society of Anesthesiologists physical status classifications II–III and a body mass index (BMI) of 40–50 kg/m2 who did not exhibit any of the following: contraindications to peripheral regional anesthesia blocks, existing infection at the block site, contraindication to regional anesthesia, history of opiate abuse, pre-existing chronic pain or cognitive dysfunction (which would impede accurate engagement with postoperative quality of recovery and analgesia assessment), refusal of the regional block, any neurological or psychological disorders, inability to cooperate, patients scheduled for concomitant laparoscopic cholecystectomy or paraumbilical hernia repair, those with a history of previous bariatric surgery or obstructive sleep apnea, patients with anatomic abnormalities at the site of injection, and those with skin lesions or a wound at the site of proposed needle insertion.
The individual indications for surgery were laparoscopic bariatric surgery, that is, sleeve gastrectomy and/or Roux-en-Y gastric bypass (RYGB) surgery.
The patients were assigned to one of the trial groups using a computer-generated random number table. Patients with even numbers were allocated into the ESP block group and those with odd numbers were allocated into the TAP block group. The patient study code number and group allocation were typed on separate pages, folded, and concealed in sequentially numbered, sealed envelopes. Block randomization in groups of six individuals was applied to ensure an even number in each group as the study progressed. The groups were named “ESP” and “TAP”. An independent third party held the randomization key. Both patients and anesthetists involved in postoperative data collection were blinded to the group to which the patients were allocated.
Upon arrival in the operating room, perioperative monitoring, which included continuous electrocardiogram (GE-Datex Ohmeda 5-lead ECG cable, USA), pulse oximetry (GE-Datex Ohmeda finger SpO2 sensor), and non-invasive arterial blood pressure (GE-Datex Ohmeda NIBP cuff), were initiated. Baseline vital signs were recorded, including non-invasive measurements of systolic, mean, and diastolic arterial pressures, and HR and oxygen saturation. After intravenous (IV) access, the patient was premedicated with metoclopramide at 0.1–0.2 mg/kg. The patient was randomly assigned to one of two groups according to the intervention used: Group A (30 patients), which received the TAP block, and Group B (30 patients), which received the ESP block.
Both blocks were performed by the primary investigator and supervised by consultant anesthesiologists who had experience in regional anesthesia and were familiar with the ESP and TAP blocks. Additionally, prior to the administration of either block, 1 mg of IV midazolam and 5 ml of lidocaine 1% infiltration were administered on both sides at the site of the block needle injection.
Ultrasound-guided blocks were performed under full aseptic conditions according to randomization before the surgery. All patients received bupivacaine 0.25% at a total volume of 40 ml regardless of the block they received. All blocks were performed with a blunted tip, 20-gauge, short bevel needle (Pajunk Sonoplex, Germany) using the same ultrasound machine (high-frequency linear ultrasound transducer, Siemens Acuson x300 3–5 MHz ultrasound), which was placed in a sterile cover.
The patients randomized to the ESP group were first placed in the prone position. The ESP block was then performed using a high-frequency linear ultrasound transducer that was placed sagittally against the target vertebral level (T5 transverse process) in the prone position and moved approximately 3 cm laterally to the spinous process. The erector spinae muscle and transverse process were then identified, and a blunted tip, 20-gauge, short bevel needle (Pajunk Sonoplex, Germany) was advanced using the in-plane approach in a cephalad-to-caudal direction through the interfascial plane between the erector spinae and the underlying transverse process under strict aseptic precautions until the tip had been advanced deep into the erector spinae muscle, as evidenced by visible hydro-dissection below the muscle plane and a 5-ml injection of normal saline to confirm the correct needle position. The block was performed bilaterally by injecting 40 ml of 0.25% bupivacaine (20 ml on each side) into the fascial plane between the deep surface of the erector spinae muscle and transverse processes of the lumbar vertebrae laterally (at the most lateral part of the transverse process).
Patients randomized to the TAP group were placed in the supine position. The TAP block was then administered using a high-frequency linear ultrasound transducer. After skin preparation and isolation, the transducer was placed 2 cm subxiphoidian and moved along the subcostal edge to identify the rectus abdominis muscle and transversus abdominis. Once these structures were identified, a blunted-tip, 20-gauge, short bevel needle (Pajunk Sonoplex, Germany) was introduced using the in-plane approach 2–3 cm laterally to the transducer under direct ultrasound visualization, and 1–2 ml of saline was injected between the rectus abdominis and transversus abdominis muscles. After confirming the correct placement of the needle and negative aspiration probe, the rest of the anesthetic was injected along the subcostal line in the TAP (20 ml 0.25% bupivacaine), and dissection of the plane was observed. The block was performed bilaterally. A total of 20 ml of 0.25% bupivacaine was injected on each side after aspiration to avoid intravascular placement.
Thirty minutes after performing each block, all patients received general anesthesia induced with fentanyl (1.5 g/kg) based on lean body weight (maximum dose of 200 g), and propofol (2 mg/kg) was administered based on total body weight. Tracheal intubation was facilitated with 0.5 mg/kg of atracurium based on the ideal body weight. Anesthesia was maintained using isoflurane in oxygen and air. Additional doses of 0.1 mg/kg atracurium were administered every 30 min. A urinary catheter was placed to control diuresis. Surgical intervention was permitted 20 min after completion of the block procedure. Volume-controlled ventilation was adjusted to maintain normocapnia. Anesthesia was maintained using 1–1.5% isoflurane in a mixture of oxygen and air (50/50) and atracurium top-ups at a dose of 0.1 mg/kg every 30 min. All participants were then administered 1 g of IV paracetamol (maximum dose of 4 g/24 h) together with 4 mg of ondansetron 10 min prior to the end of surgery for postoperative nausea and vomiting prophylaxis.
After skin closure, the inhaled anesthesia was discontinued and muscle relaxation was reversed with IV atropine (0.02 mg/kg) and neostigmine (0.05 mg/kg) after the return of spontaneous breathing. Patients were transferred to the post-anesthesia care unit (PACU) for 60 min for complete recovery and monitoring.
If at any point hypotension occurred (defined as a decrease in mean arterial pressure > 25% from the baseline value or systolic arterial pressure of 100 mmHg), it was treated with 5 mg of IV bolus ephedrine, which was repeated every 3 min until the hypotension resolved. Bradycardia (defined as an HR of 40 beats/min) was treated with intravenous atropine (0.5 mg).
In the PACU, the visual analog scale (VAS) score was assessed 15 min after extubation by the attending anesthetist. When the score was ≥ 4/10, rescue analgesia in the form of nalbuphine 0.1 mg/kg (individual dose not to exceed 20 mg and a maximum dose of 50 mg/24 h) was administered. Another dose of nalbuphine 0.1 mg/kg was given in the PACU to patients with a VAS score > 4 30 min after the first dose.
After discharge from the PACU, the analgesia plan was intravenous paracetamol (1 g/8 h), nalbuphine (0.1 mg/kg/8 h) if the VAS score was ≥ 4, and ketorolac as a second rescue analgesia (0.5 mg/kg/6 h) as long as the pain score remained ≥ 4 (reassessment was done by the nurses in the ward 30 min after administration of the first rescue analgesia).
The primary outcome was the analgesic efficacy of the ESP block versus the TAP block, as assessed by the mean VAS score in the first 24 h postoperatively.
Secondary outcomes were as follows: the time required for a successful block (measured as the time from initiation of ultrasound scanning to completion of the block on both sides), incidence of complications, time to first rescue analgesia, time to first flatus or stool passage, and total opioid consumption.
Statistical analysis
In a previous study [9], the mean VAS in the TAP block group in the first 24 h was 3.34 ± 0.66 at rest and 4.46 ± 0.85 for dynamic postoperative pain. We calculated the sample size that could detect a mean difference of 20% between the study groups. MedCalc software (version 14; MedCalc Software Bvba, Belgium) was used to calculate the sample size. Fifty patients (25 patients per group) were estimated to have a study power of 80% and an alpha error of 0.05. This number was increased to 60 patients (30 per group) to account for possible dropouts. Data were coded and entered using the Statistical Package for the Social Sciences (SPSS) (version 26; IBM Corp., USA). Data were summarized using the mean ± SD for quantitative variables and frequencies (number of cases) and relative frequencies (percentages) for categorical variables. The Shapiro-Wilk test was used to assess data normality. Comparisons between groups were performed using the unpaired t-test. To compare categorical data, the chi-squared test was used. Statistical significance was set at P < 0.05.
Results
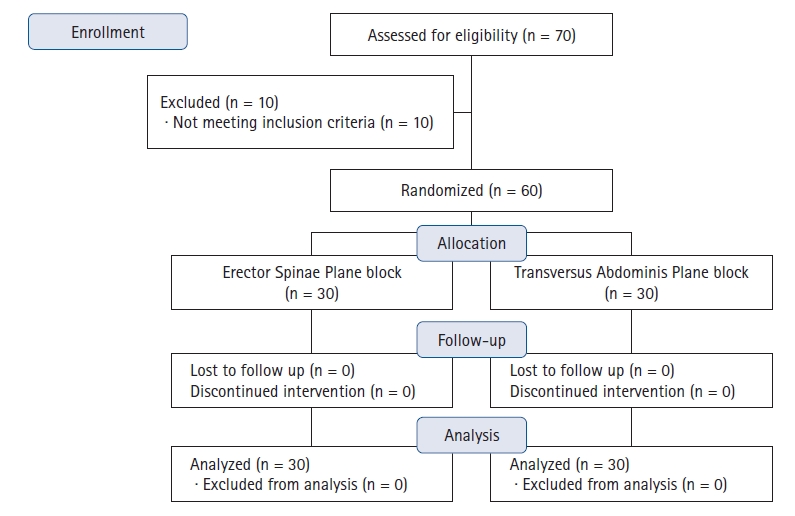
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram for this trial is shown in Fig. 1. Seventy patients were initially screened for eligibility, 60 of which met the inclusion criteria and were randomly assigned to receive either the ESP block or TAP block. All enrolled patients were followed up successfully, and no patients were lost to follow-up.
Patient and surgical baseline data
Baseline patient data and the type of bariatric surgery were comparable between the groups apart from the STOP BANG score, which was higher in ESP compared TAP (P = 0.035) (Table 1).
Profile of monitoring and adverse events
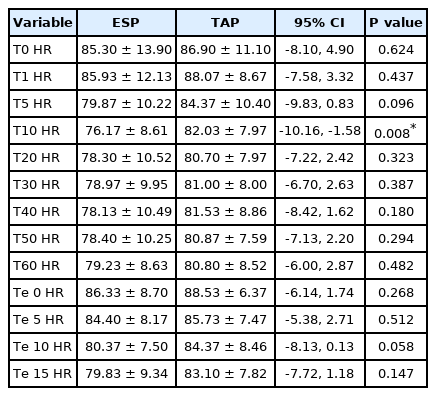
Patient hemodynamics for each group are shown in Tables 2 and 3. There were no statistically significant differences in the patients’ hemodynamics between the groups. In addition, patients who received the TAP block at 10 min after induction showed a significantly higher heart rate than those who received ESP at the same time point (P = 0.008). Adverse events were reported as incidents over the 24-h observation period of the study. There were no adverse events related to the regional anesthesia techniques used in either group.
Pain assessment based on the VAS score (primary outcome)
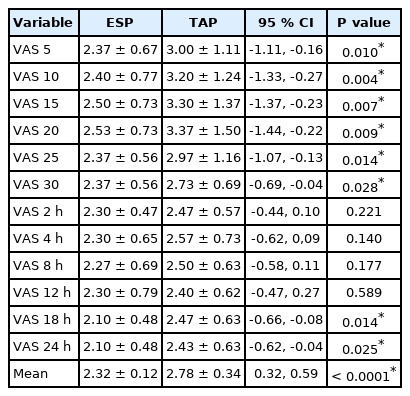
The patients’ assessment of pain according to VAS scores after extubation is shown in Table 4. A statistically significant difference was found between the two groups, with a higher VAS score in the TAP than in the ESP block group in the period between 5 min to 30 min after extubation and at 18 and 24 h post-extubation, with a higher mean VAS score in the TAP block group in the first 24 h postoperatively compared to the ESP block group (P < 0.001).
Secondary outcomes
Secondary outcomes, such as the time to first rescue analgesia, total opioid consumption, and time of peristalsis between the two groups are shown in Table 5. The results were statistically significant for intestinal peristalsis, which was delayed more with the TAP than with the ESP block (P < 0.001). Additionally, the time to first rescue analgesia was longer with the ESP than with the TAP block (P = 0.001), the total nalbuphine consumption was lower with the ESP than with the TAP block (P < 0.001), and the time required for a successful block was higher with the TAP than with the ESP block (P = 0.001).
Discussion
This is the first randomized controlled study comparing the ESP and the TAP blocks in patients with obesity undergoing laparoscopic bariatric surgery. The main finding of our study was that the ESP block showed a better analgesic effect, with lower postoperative opioid consumption than the TAP block. Moreover, those in the ESP block group regained intestinal function earlier than those in the TAP block group, as indicated by the time to flatus or stool passage; however, the results regarding intraoperative hemodynamics were similar between the groups.
Consistent with our findings, Altıparmak et al. [8] found that an ultrasound-guided ESP block reduced postoperative tramadol consumption and pain scores more effectively than did the oblique subcostal TAP block after laparoscopic cholecystectomy. In addition, the results of a study comparing the ESP and the TAP blocks in patients with obesity undergoing sleeve gastrectomy were consistent with our results regarding the superior analgesic effect of the ESP block [9]. The most striking difference between these studies and ours was that in these studies, the feasibility of each block (especially in challenging populations such as patients with obesity) was not investigated. Additionally, the authors used tramadol and pethidine as postoperative pain control modalities rather than nalbuphine, which is considered to have a potent analgesic effect with a low incidence of adverse events [10].
According to another study [11], the TAP block and trocar site infiltration provide comparable pain relief in patients undergoing laparoscopic bariatric surgery. Because of its faster application and fewer side effects, we believe that the ESP block could be a potent, time-saving, and highly successful pain control method in patients undergoing laparoscopic bariatric surgery. This could result in a faster application, similar to trocar site infiltration, with a better analgesic effect.
On the other hand, Mittal et al. [12] found that the ultrasound-guided TAP block is a feasible, minimally invasive technique and can be part of effective multimodal analgesia in morbidly obese patients undergoing bariatric surgery. However, in that study, TAP blocks were compared with a control group with only systemic analgesia. In contrast, our study compared the TAP block to the ESP block and found that both were effective, but the ESP block showed a more potent analgesic effect. Our study also showed that less time was needed to perform the ESP block than the TAP block, and this time difference was clearly notable in our study due to the higher mean BMI in such a challenging population.
Additionally, Keller et al. [13] conducted a pilot study on the feasibility and learning curve associated with TAP blocks in patients with obesity and showed that novices reach appropriate time to perform a successful block with progressively less coaching to place TAP blocks safely and efficiently. However, the duration of the procedure can be prolonged in patients with extreme BMIs and prior abdominal surgery, potentially resulting in the need for additional coaching to facilitate placement. When we compared the time required for a successful TAP block in Keller et al.’s study with the time required to perform a successful ESP block in our study, it was clear that the ESP required less time than the TAP block, not to mention the patients’ BMIs were clearly higher in our study.
Our study also showed, for the first time, a difference in the time to flatus or stool passage between the two groups, which could be explained by the sympathetic blockage associated with the ESP block. While the ESP block has been used to treat paralytic ileus [14], to the best of our knowledge, no previous studies have investigated whether the time to regain proper intestinal function is shorter with this block, which could result in a shorter hospital stay.
Our study also had some limitations. First, the results of intraoperative hemodynamics may be affected by other variables, such as duration of surgery, surgeons’ skills, and manipulations during surgery such as bougie insertion. Furthermore, there were no data available to compare preoperative baseline pain and anxiety scores with postoperative scores; however, the VAS score was applied equally to both randomized groups. In addition, there was heterogeneity in the type of surgery between the two groups, with a higher percentage of patients undergoing RYGB compared to sleeve gastrectomy surgery in the ESP group than in the TAP group; however, we believe that it did not have an impact on the results, as the difference was not statistically significant, and the resemblance to the trocar site insertion in RYGB and sleeve gastrectomy surgeries would cause similar degrees of postoperative pain.
In conclusion, the bilateral ESP block is a more feasible and effective method for intra- and postoperative analgesia in patients undergoing laparoscopic bariatric surgery than the bilateral TAP block.
Notes
Funding
This study was funded by the Department of Anesthesia, Surgical ICU, and Pain Management, Faculty of Medicine, Cairo University, Cairo, Egypt (No. MD-250-2020).
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Mohamed Elshazly (Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing)
Yasser Mohamed EL-Halafawy (Methodology)
Dina Zakaria Mohamed (Methodology)
Khaled Abd El Wahab (Investigation; Writing – original draft; Writing – review & editing)
Tamer Mohamed Kheir Mohamed (Data curation)