Postoperative opioid consumption data repository incorporated in electric medical record based on PCA device data extraction program
Article information
Pain is often referred to as the ‘fifth vital sign’ and is a crucial element of contemporary patient care. It is increasingly recognized that assessment and adequate control of pain are just as important as the other vital signs. In particular, the importance of postoperative analgesia cannot be overstated since it is known to be related to various postoperative complications and the quality of recovery. Therefore, the issue of postoperative analgesia is an indispensable and constant mission for perioperative care physicians. Patient-controlled analgesia (PCA) is one of the valuable tools providing optimal postoperative analgesia. PCA devices allow the patient to self-administer small boluses of analgesic to relieve pain [1]. Use of PCA has been recommended over intermittent bolus dosing of opioids initiated by health care providers [2].
Several electrical or elastomeric PCA devices are being widely used. Among these, some electrical devices incorporate settings to calibrate various parameters, such as bolus dose, lockout interval, background infusion rate, dose limits, and loading dose. In addition, the detailed device usage data can easily be stored and extracted via designated software. Here we briefly introduce the integrated data management system (Dr. PCA, Data repository for postoperative clinical audit), which is incorporated into the electronic medical record (EMR) system at our institution (Supplemental File 1).
At our institution, the PCA device is returned to a designated place for data extraction upon completion of its use. Using the dedicated software of our PCA device installed in computers connected to the hospital network, PCA data are extracted and the detailed log records are automatically saved to a repository server. For security, the raw data is stored in a separate server, and only the processed relevant information is retrieved from the EMR. The software of the PCA device generates data based on the event logs, which makes it possible to calculate the operating time of the device and to process the accumulated data over time.
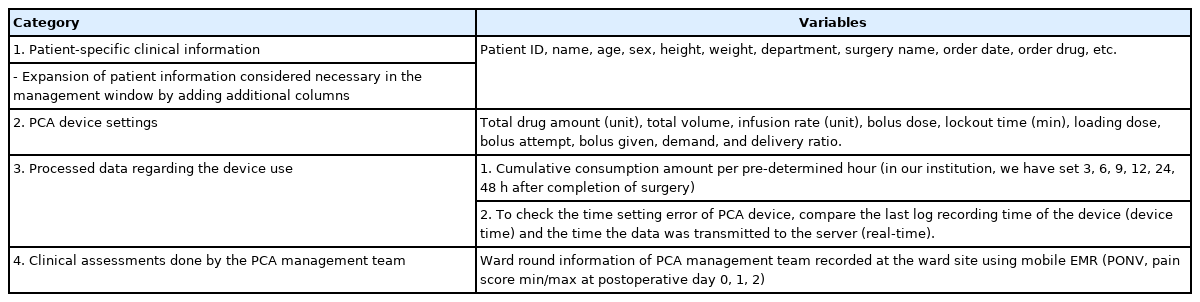
The data management system consists of mainly four types of information (Table 1):
1. Patient-specific clinical information
2. PCA device settings
3. Processed data regarding the device used
4. Clinical assessments done by the PCA management team
As noted above, the system integrates not only the information extracted from the device but also the data from the PCA management team. In Korea, the operation of the ‘PCA management team’ is one of the items of interest for quality assessment of anesthesia practice. The clinical assessment at our institution routinely includes pain score (numerical rating scale – maximum and minimum) and adverse effects relevant to PCA (e.g., postoperative nausea and vomiting, somnolence, and dizziness). This is an efficient means to enable clinicians to evaluate their practice and gain feedback. Moreover, as this system regularly operates and consistently stacks comprehensive ‘pain-related’ outcomes, it can enable systematic evaluation and modification of a certain protocol or regimen for postoperative analgesia.
Alongside the ‘opioid crisis,’ there are increasing concerns about chronic opioid use beyond the acute phase of the postoperative period [3]. Therefore, minimizing or optimizing perioperative opioid use is also a noteworthy issue. The establishment of a model to deal with this issue may require a large dataset of detailed information regarding postoperative analgesia. Therefore, the establishment of a system for accumulating relevant data is a key imperative. Analysis of the temporal patterns of analgesic demand in individual patients may provide valuable insights into perioperative analgesia. Resolution of the concerns about device hacking by wireless network connection will facilitate remote monitoring of real-time usage patterns and pain assessments. This would ultimately enable the provision of more advanced acute pain services. In addition, the use of big data technology to analyze PCA data may help predict opioid use for individual surgery patients [4].
Notes
Funding
This work was supported by research funding from Chungnam National University Hospital Research Fund (2021-CF-035).
Conflicts of Interest
No potential conflict of interest relevant this article was reported.
Author Contributions
Chahyun Oh (Conceptualization; Project administration; Writing – original draft; Writing – review & editing)
Boohwi Hong (Conceptualization; Funding acquisition; Project administration; Writing – original draft; Writing – review & editing)
Gwanhoon Kim (Resources; Software; Writing – review & editing)
Seok-Hwa Yoon (Supervision; Writing – review & editing)
Yong-Sup Shin (Supervision; Writing – review & editing)
Supplementary Material
Workflow of data repository of patient controlled analgesia device and clinical information