Different perspectives for monitoring nociception during general anesthesia
Article information
Abstract
Safe anesthesia is achieved using objective methods that estimate the patient’s state during different phases of surgery. A patient’s state under anesthesia is characterized by three major aspects, which are linked to the main effects produced by each of the families of anesthetic agents administered: hypnosis, analgesia, and muscular relaxation.
While quantification techniques designed to assess muscular relaxation under neuromuscular blocking agents have a relatively long history with a high degree of standardization and understanding (e.g., the train-of-four), the knowledge and techniques used to the depth of hypnosis assessment suffer from a lesser degree in both standardization and interpretation due to brain complexity.
The problem of standardization and interpretation in the analgesia and nociception assessment increases since it involves more systems, the central nervous system, and the autonomic nervous system.
This helps to explain why there are multiple a priori valid approaches to develop nociception monitoring from different interpretations and physiological bases of noxious stimuli processing. Thus, in this review, the current monitoring technologies clinically available for estimating a patient’s nociception under general anesthesia are described.
Introduction
Monitoring anesthesia
Despite the benefit of the administration of general anesthetics in patients undergoing surgery, their high risks have been recognized, with numerous potential adverse effects [1].
Multi-modal and balanced anesthesia methods refer to all agents and techniques that interact with different components of anesthetics, from hypnosis and analgesia to muscular relaxation, while maintaining homeostasis and preventing undesirable autonomic reflexes [1]. For procedures involving anesthesia to be safe, it is crucial that objective methods exist that estimate the state of each anesthesia component along the different phases of the surgical context to provide the practitioner with adequate information for deciding the appropriate actions to take to achieve the desired state for a given patient.
Hypnosis, analgesia, and muscular relaxation depict distinct aspects of a patient’s state, although none are fully independent. Although anesthetic drugs specifically target an anesthetic component, they might also influence other components either alone or through interaction with other agents (Fig. 1) [1–3]. For example, the hypnotic effect of propofol is potentiated by μ-agonist opioids, lowering the propofol effect-site concentration needed to achieve loss of consciousness and a loss of response to commands and pain [4,5]. Monitoring technologies for hypnosis and nociception should reflect such synergy to help practitioners balance anesthesia.
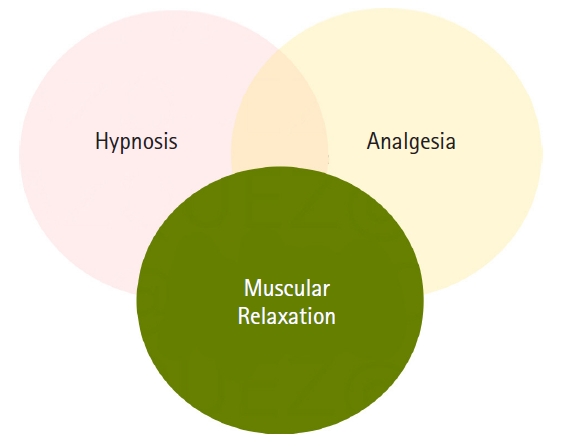
General anesthesia components relationship scheme. The close relationship between hypnosis and analgesia, which is commonly synergetic, is symbolized by a translucent intersection. However, the undesired effect of muscular relaxation masking and hindering the assessment of hypnosis and analgesia is symbolized with an opaque color covering a complete observation (assessment) of the other two components.
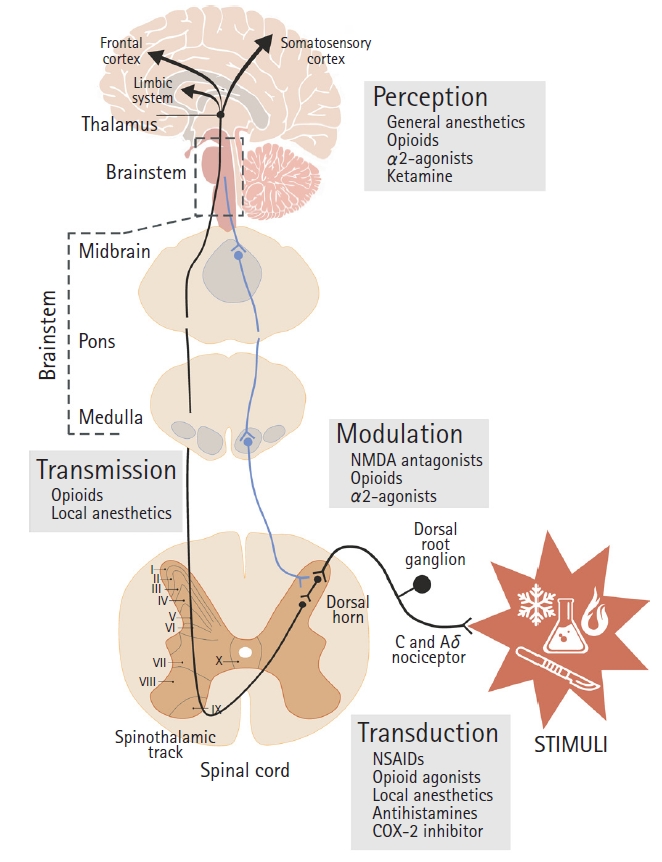
Simplified illustration of the general pathway of nociception. Anterolateral ascending spinothalamic nociceptive transmission path (black line) and descending modulatory path (blue line). Locations of action of the nociception processes (transduction, transmission, modulation, and perception) and most noteworthy related substances. NSAID: non-steroidal anti-inflammatory drug, NMDA: N-Methyl-D-aspartate receptor.
Fig. 1 shows the close and commonly synergistic relationship between hypnosis and analgesia as a translucent intersection, while the relationship between muscular relaxation and hypnosis and analgesia is pretty different. While hypnotic and analgesic agents promote some muscle relaxation, muscular activity per se has no significant effect on hypnosis or analgesia. In addition to the practical benefits of neuromuscular blocking agents (NMBAs) during surgical procedures, the drawbacks of muscular relaxation reside in the suppression of the patient’s movement response, hindering a full assessment of the hypnotic and analgesic effects. This effect of muscular relaxation masking the assessment of other components is shown in Fig. 1, with superimposed opaque intersections over the other components.
Neuromuscular monitoring
The study of muscular relaxation induced by NMBAs has a comparatively long history, with many quantification techniques and a relatively high standard of agreement regarding their use, extensions, and limitations. Neuromuscular monitoring (NMT) is crucial every time NMBAs are used, especially to estimate when the neuromuscular blockade is sufficiently reversed [6]. The principle of NMT relies on peripheral nerve stimulation-response quantification. Stimulation patterns and measurements can vary from single-twitch to train-of-four, tetanic and post-tetanic counts, and double-burst stimulation. Despite the variety of modalities, all approaches rely on the same principle.
Hypnosis monitoring
Hypnosis monitoring has been increasingly used since the mid-1990s. Hypnosis assessment technologies rely on electroencephalography (EEG) analysis; however, they lack a gold-standard definition. Most of these technologies rely on correlating distinct EEG patterns to the concentrations of different agents and qualitatively evaluating clinical signs using sedation scales, such as the observer’s assessment of alertness/sedation scale (OASS). These technologies are more complex than NMT owing to the increased complexity of such estimations. Several EEG features and algorithms have been used to define depth of hypnosis indices [7,8], and despite the variety of estimation methods, their concordance is high [9]. Indeed, these indices have been used to enhance an optimization of anesthesia drug consumption [10–12], prevent awareness with recall events due to underdoses [13–15] and excessive concentrations (overdoses), and improving patients’ outcomes [16–21].
The need for nociception assessment
Assessing the level of analgesia in the perioperative context essentially refers to an analysis of physiological neural encoding and processing of noxious stimuli. The goal of monitoring nociception (from Latin noci, meaning harm or injury) is to objectively quantify the responses induced by surgical stress to help to maintain a nociceptive-anti-nociceptive balance [22,23].
From the arsenal of anesthetic agents available for general anesthesia (GA) and in the intensive care unit, opioids play an essential role in the management of nociception. Opioids have several benefits, including a reduction in preoperative pain and anxiety, decreased somatic and autonomic responses to airway manipulations, improved hemodynamic stability, lower dose requirements for inhaled agents, and immediate postoperative analgesia [1]. However, opioids are also associated with many well-known adverse effects. Excessive administration of opioids increases the frequency of side effects, such as nausea, vomiting, respiratory depression, opioid-induced hyperalgesia, and the potential for opioid addiction [24–27].
Anatomy and physiology of nociception in a nutshell
The complexity of nociception processing comes from the multiple complex systems involved in the processing of noxious stimuli, including both the autonomic nervous system (ANS) and the central nervous system (CNS), see Fig. 2. Nociception involves four major processes: transduction, transmission, modulation, and perception.
The complexity of nociception begins with the nature of the stimuli, where differences in nociception processing depend on the type of sensory modality involved (Fig. 2), meaning whether stimuli are mechanical (pressure, pitch), thermal (heat), or chemical, and their specific pain receptors or nociceptors. In addition, processing depends on the location of the stimuli, from the cutaneous nerves to the visceral or deep musculoskeletal tissues. These differences in sensory modalities and locations at the transduction level influence nociceptive processing and perception. Focusing on the ascending pain pathway, the nociceptive message is coded in the pattern and frequency of action potentials triggered by different chemicals released by injured cells (e.g., prostaglandins) and transmitted to the spinal cord through the axon of the primary afferent nociceptor (first-order cell). This neuron has its cell body in the dorsal root ganglion, with one axon branching out to the periphery and another into the spinal cord, ending near second-order nerve cells in the dorsal horn of the gray matter (substantia gelatinosa) that project over the anterolateral quadrant of the spinal cord to the brain stem and thalamus. Primary afferent nociceptors release transmitter substances to the spinal terminals (substance P), stimulating second-order pain transmission cells. Despite this, there is a variable relationship between nociceptor input and perceived pain intensity. In general, the intensity of the stimuli is proportional to the frequency of the nociception discharges along the ascending pathway. Once nociceptive signaling reaches the thalamus, it is projected to widespread areas of the forebrain through third-order neurons, from the somatosensory cortex and limbic system to the frontal cortex.
Nociceptive signal transmission is regulated by the activity transmitted through the descending pathway through the midbrain, crossing the medulla and ending at the dorsal horn, at a serotonergic-noradrenergic neuron that inhibits the release of substance P between the first-order and second-order neurons of the ascending path, and stimulating a nearby opioid-interneuron that additionally releases an endogenous opioid (enkephalin), which helps inhibit the pre- and post-synaptic exchange of substance P.
Opioids act on both the brain and the spinal cord, stimulating the activity of the descending inhibitory pathway from the midbrain to the dorsal horn. For instance, remifentanil, a µ-receptor agonist [28], modulates nociceptive transmission and processing where this receptor is distributed, which may be in the brain at the cerebral cortex (upper part of layer V-VI) or throughout the spinal cord (primarily confined to laminae I–II, dorsal horn) and the peripheral nervous system [29].
This brief summary of the nociception system disregards a much deeper description of the mechanisms involved in nociception processing, such as differences in sensory cell types and characteristics, other relevant neurotransmitters, and interactions among various other factors [30–32]; however, this summary is meant as a brief explanation of the physiological basis for the a priori adequacy of the different existing nociception monitoring technology approaches.
Nociceptive information is communicated to the ANS and CNS via the spinal cord, brainstem, and thalamus. It is important to note that the different monitoring technologies are not explicitly related to direct measurements at certain points of the pain pathway, but rather to the responses to noxious stimuli at the level of the ANS and CNS, such as heart rate, blood pressure, skin conductance, and EEG.
Nociception monitoring
Traditional cardiovascular parameters and clinical signs under some circumstances provide valid clinical criteria for inadequate anesthesia, such as systolic blood pressure 15 mmHg above baseline and heart rate > 90 beats/min as well as other autonomic signs (e.g., sweating, flushing, or lacrimation) and somatic responses (e.g., body movements, swallowing, coughing, grimacing, or eye movements) [33]. However, these parameters and signs generally have low sensitivity and specificity for nociception because they can also be affected by anesthetics (e.g., propofol affects blood pressure, ephedrine affects heart rate) and other factors related to the surgical procedure (e.g., absence of heartbeat under cardiopulmonary bypass). Therefore, nociception monitoring technologies are needed that complement the traditional clinical criteria.
Just as with hypnosis, there is no gold standard to measure nociception given the many interacting complex systems and mechanisms involved [30,33,34] along with inherent subject variability. However, some crude but objective approaches for estimating nociception may be sufficiently valid to help practitioners in clinical decision-making.
In the following sections, we will describe the main features of the monitoring systems that are available targeting nociceptive state inference according to the physiological system targeted (CNS, ANS, spinal reflex) and their related biosignals (EEG, electrocardiography [ECG], electromyography [EMG], plethysmography, pupillometry, and skin conductance). The following technologies will be described:
CNS-based monitoring
- Conox monitoring: qNOX
- Entropy monitoring: response entropy
ANS-based monitoring
- Pupillometry
- Analgesia nociception index monitor
- Surgical pleth index
- Nociception level index
- Skin conductance
Spinal reflex-based monitoring
- Nociceptive flexor reflex (NFR)
CNS-based monitoring
The important role of nociception assessment from brain activity measurements has been shown by Lichtner et al. [35], who reported that in patients administered remifentanil nociceptive-related activations, observed under fMRI, persist despite a lack of clinical responses. Furthermore, those dose-dependent bold-fMRI signals evoked by noxious stimuli have been described in multiple brain regions, especially in frontal areas.
Using current anesthesia depth monitoring technologies, EEG signal information can be used to evaluate the patient’s hypnotic state under GA. Additionally, multiple studies have shown distinct EEG components modulated by noxious stimuli-related information that can be used to detect situations of stress and help in the management of intraoperative analgesia administration, such as beta and delta arousals and alpha dropouts, among other changes [36–39].
However, EEG analysis and interpretation, either for monitoring hypnosis or nociception, may be hampered by the presence of EMG signals. Depending on the operative context, the presence of EMG on EEG may produce a potential bias of EEG-derived indices; however, modern EEG processing algorithms offer improved suppression of EMG signals compared to those implemented in earlier monitors. With EEG monitoring, it is important to interpret the EEG indices along with EMG signals, as EMG signals can also be an early indicator of arousal or nociception.
Entropy monitoring: response entropy index
The spectral entropy monitor (GE Healthcare, USA) is a two-index EEG-based monitor. One index focuses on describing the state of hypnosis (state entropy, [SE]) and the other evaluates the patient’s response to noxious stimuli (response entropy, [RE]). Essentially, the spectral entropy is computed over the frequency range of 0.8 to 32 Hz to define the SE (EEG-dominant part) and from 0.8 to 47 Hz to define the RE, which includes the EEG-dominant and EMG-dominant parts of the spectrum [40]. The entropy measurements are then scaled into two different unitless scores, from 0 (very deep anesthesia) to 91 (awake state) for SE and from 0 to 100 for RE.
Under GA with propofol and remifentanil, high RE values (> 55) before stimulation increase the risk of a motor response. However, lower values do not prevent a response when the opioid concentration is insufficient, despite adequate hypnosis [41].
Entropy-guided anesthesia during propofol-remifentanil GA has resulted in fewer unwanted patient responses compared to standard practice along with a reduction in opioid consumption [42]; however, no differences have been seen regarding recovery, hemodynamic parameters, or postoperative outcomes.
The SE-RE difference appears to be a potential proxy for facial EMG activity, and thus might be useful for assessing nociception during surgery [43]. Its use for controlling the remifentanil dose has been suggested [44].
Conox monitoring: qNOX index
Similar to the spectral entropy monitor, the Conox monitor (Fresenius Kabi AG, Germany) integrates two EEG-based indices. The qCON index is an indication of the patient’s level of consciousness, and the qNOX index can be used to gauge the probability that a patient will respond to noxious stimuli. Similar to the qCON index, which links different EEG spectral components to distinct aspects of hypnosis (loss of consciousness event, hypnotic concentrations, level of alertness/sedation scales) using a quadratic model, the qNOX index integrates the spectral components into an equivalent model that best predicts whether a patient will respond to noxious stimuli [45]. The likelihood of movement response to external stimuli is described on a scale ranging from 0 to 100. The recommended qNOX index values for GA are between 40 and 60, where a value > 60 corresponds to a high probability of response to external noxious stimuli and a value < 40 corresponds to a low likelihood of response. If the qCON and qNOX values equal 0, this indicates an isoelectric EEG signal, and consequently, a burst suppression ratio of 100%.
In one study of 60 patients, significant increments in the qNOX values pre-and post-noxious stimuli (LMA insertion, tracheal intubation, and laryngoscopy) were found; however, the remifentanil or propofol effect-site concentrations were not correlated with whether the patient moved in response [45].
The qCON and qNOX indices behave differently for detecting loss of consciousness and loss of response to nociceptive stimulation. In a study of 140 patients scheduled for propofol-remifentanil GA, the qCON index was found to be better for predicting loss of consciousness, such as loss of verbal command and eyelash reflex, than the qNOX index, while the qNOX index had a better predictive value for response to noxious stimuli [46]. Furthermore, the qNOX index increased faster at the end of surgery, leading to the hypothesis that the response to stimuli is recovered faster than the consciousness recovering. Thermoregulatory processes are essential for the activation of analgesic mechanisms, given the physiologically strong negative association between nerve conduction velocity and temperature, in addition to having significant repercussions on the pharmacological dynamics of analgesic drugs (decreased clearance rates with a subsequent increase in effect-site concentrations). Based on the hypothesis that deep hypothermia produces considerable effects on a patient’s analgesia and hypnosis levels, in one study, 39 patients who underwent elective on-pump coronary artery bypass graft surgery under hypothermia were monitored using the bispectral index (BIS) and Conox monitors. While the hypnotic indices (BIS, qCON) showed significant but weak correlations with respect to the temperature, the qNOX index showed the strongest correlation [47] not only for population behavior, but more importantly, for the prediction of each individual patient using a linear mixed-effect model for temperature with the patient as a random factor (BIS: R2=0.06, P < 0.05; qCON: R2=0.29, P < 0.001; qNOX: R2=0.74, P < 0.001).
ANS-based monitoring
Pupillometry
The pupillometric assessment of analgesia and nociception relies on portable measurements of pupil diameter response systems that are based on the idea that pupil constriction and dilation is controlled by a sympathovagal balance, since the pupillary muscles are innervated by both the sympathetic and vagal nerves [48]. Of the different infrared pupillometers used for assessing nociception, such as the ANeurOptics PLR-100 (NeurOptics, USA), the Algiscan system (IDMed, France) is unique in that it has an integrated electrical stimulation unit, which allows for easy operation under four different modes. In the first operation mode, changes in pupil size are evaluated in response to noxious stimuli (such as incision or electrocautery) over a 60 s time frame. The second mode is used to measure the changes in diameter after exposure to a 1-s flash of light (320 lux). The third and fourth modes, named the tetanus and pupillary pain index (PPI) modes, correspond to the elicited pupil changes after distinct controlled electrical stimulations are applied to the ulnar nerve. Each mode is different in terms of the stimulation frequency pulse, duration time, and type of amplitude stimulus (constant or variable; rate change 10 mA/s to a maximum of 60 mA). The PPI mode is defined as a dimensional index ranging from 0 to 10, where lower values represent lower pupil reactivity and thus deeper analgesia, and higher values (PPI > 7) indicate insufficient analgesia.
Pupil diameter reactivity has been shown to correlate with remifentanil effect-site concentrations [49], intraoperative nociception response predictions [50-53], and postoperative pain assessments [54–56]. Pupillometry has been shown to demonstrate a faster response to stimuli than heart rate and arterial pressure and allows for the prediction of the analgesic state before stimulation [49,57]. In one study, pupillary dilation after standardized tetanic stimulation was influenced by propofol concentrations in patients with constant effect-site infusion of 1 ng/ml of remifentanil. This suggests that pupil reactivity (in this case, the stimulus elicited) appears to also be influenced by the hypnotic level [58]. Further research is required to evaluate the effects of hypnotics on pupillometry and other potential confounding factors. The main drawbacks are the discontinuous monitoring and need for careful corneal care, opening the eyelid in each of the multiple perioperative measurements required to follow patient changes. Measurements might also be affected by neostigmine, pupillary diseases (Horner and Holmes-Adie syndromes), and blindness. Additionally, care must be taken regarding ambient light conditions.
Analgesia nociception index monitoring
The analgesia nociception index (ANI) monitor (MDoloris, France) evaluates the parasympathetic response reflected in the ECG during GA. This dimensionless index based on heart rate variability measures the influence of the parasympathetic system on cardiac rhythm during respiration calculated using high-frequency band-pass filtered R-R series (between 0.15 and 0.4 Hz). It ranges from 0 (maximal nociception) to 100 (maximal analgesia) [59,60]. The ANI mean normal values fluctuate between 50 and 70, where an ANI < 30 for longer than 5 min indicates analgesia underdosing and an ANI > 70 indicates analgesia overdosing.
The ANI has been investigated in both conscious and anesthetized subjects. Boselli et al. [61] reported that dynamic variations in the ANI in patients under desflurane-remifentanil GA, rather than static ANI values, were significantly predictive of hemodynamic reactivity [61]. The ANI, pupillometry, and surgical pleth index (SPI) were superior at detecting painful stimulation compared with traditional hemodynamic parameters, and the performance was attenuated by increasing remifentanil dosages. However, baseline values showed significantly lower prediction probabilities for nociceptive responses [62].
An observational study of children aged 2 to 12 years showed changes in the ANI 5 min before and after the surgical incision, where hemodynamic parameters were found to be of low or no predictive value for detecting noxious stimuli [63]. Further research using the ANI is needed to evaluate its relationship to opioid concentrations, as well as its applications outside of GA, such as regional blocks or conscious sedation. Caution must be taken when using the ANI monitor or other ANS-based monitors; since agents acting on the ANS, such as ephedrine and atropine, may affect the index score [64]. The ANI is thus not reliable for approximately 10 min after ephedrine administration and 20 min after atropine administration. This raises concerns about other agents and drug combinations that affect the ANS, such as beta-blockers. Further study is therefore necessary.
While the ANI indicates noxious stimulations during GA anesthesia, its interpretability might be limited given the large associated interindividual variability and low reproducibility [22,65]. Finally, the ANI index may also not be useful during intubation when the patient is apneic.
Plethismography-based monitoring
The SPI (GE Healthcare, Finland) relies on plethysmography pulse-wave changes provoked by noxious stimuli: a sympathetic response to peripheral vasoconstriction and cardiac autonomic tone. The SPI is computed as the normalized heartbeat interval (HBInorm) and plethysmographic pulse wave amplitude (PPGAnorm): SPI = 100 – (0.7 × PPGAnorm + 0.3 × HBInorm) [66]. This unitless score ranges from 0 to 100, with lower values indicating deeper analgesia. An SPI > 50 is considered inadequate analgesia.
The SPI responds to remifentanil concentration changes and is higher at lower remifentanil concentrations. Additionally, the SPI reacts to surgical nociceptive stimuli and analgesic drug concentration changes during propofol-remifentanil anesthesia, where the SPI increases at skin incision and remains high during surgery than before surgery [66]. SPI-guided anesthesia has been reported to result in lower opioid [67] and propofol [16] consumption with more stable hemodynamics, a lower incidence of unwanted events, and shorter arousal times.
The physiological basis for the SPI is generally not valid because the SPI is not interpretable for postoperative pain assessment in conscious subjects [68]. Furthermore, this biosignal might be significantly affected by agents that act on hemodynamics as well as inotropic and chronotropic agents, among other factors. Surprisingly, SPI also does not appear to be valid in children, where SPI-guided analgesia leads to less fentanyl consumption but more postoperative agitation and higher analgesia requirements compared to conventional practice [69]. This may be due to both blood vessel distensibility and the higher heart rates at baseline in children versus adults, or it might suggest that opioid levels used in standard practice in children are closer to the minimum acceptable concentration threshold than the levels used for adults. This situation suggests a need to redefine the index for children. While the margin for reduction in the consumption of some agents may be larger in adults, this is not only related to nociception, but also to the influence of anesthetic agents on hemodynamic variables. In this sense, neither skin conductance nor SPI monitoring reliably predicts changes in plasma stress hormone levels (adrenaline, noradrenaline, adrenocorticotrophic hormone, and cortisol) throughout the intraoperative period [70].
Nociception level index
The PMD100 monitor (Medasense Biometrics, Israel) includes the nociception level (NOL) index. The NOL index is a score that integrates different parameters from multiple biosignals, reported as a function of heart rate variability (at the 0.15 to 0.4 Hz band power), plethysmograph wave amplitude, and skin conductance [71,72]. All biosignals are collected with a finger probe placed on the index finger of the right hand containing photoplethysmographic and galvanic skin sensors, a skin temperature sensor, and a three-axis accelerometer. The NOL is a unitless index, updated every 5 s, that ranges from 0 to 100, where lower values indicate lower sympathetic activation, deeper analgesia (recommended values are between 10 and 25 for maintenance). Under GA, the NOL index has been reported to have a higher intraoperative sensitivity and specificity than heart rate and mean arterial pressure (MAP) in predicting responses to noxious stimuli, such as intubation, incision, and tetanic stimulation [72,73]. NOL-guided analgesia during major abdominal surgery has been reported to result in 30% less remifentanil consumption [74]. Peristimulus changes in the NOL have also been reported to correlate with the remifentanil dosage [75]. In abdominal surgery with fentanyl/sevoflurane, despite the non-significant differences in fentanyl and morphine consumption after surgery, an improvement in the postoperative pain scores has been seen in patients receiving NOL-guided fentanyl administration compared to patients receiving the standard heart rate and MAP-guided fentanyl administration [76]. However, in this study, no clear differences were observed between the NOL values during NOL-guided administration and standard care [76], as essentially all were within or below the recommended maintenance values of 10–25. This suggests that the NOL index scale definition is not very well-adjusted for NOL-guided administration because the recommended value range (10–25) is relatively narrow and largely not centered within the whole dynamic range (0–100). Thus, the NOL index should be rescaled to offer greater sensitivity and dynamics.
The role of temperature and accelerometry as side parameters or modulators of the NOL index in the PMD100 monitor is also not clear. Furthermore, research on the NOL index under other important settings, including regional anesthesia, combined regional anesthesia and local analgesia, and sedation, is needed.
Skin conductance
The skin conductance algesimeter (Med-Storm, Norway) system monitors the skin galvanic response as a proxy for sympathetic nervous system activity, where increments in sympathetic activity result in filling of the palmar and plantar sweat glands. Skin conductance measurements rely on sympathetic terminals encircling sweat glands that are innervated by postganglionic sympathetic neurons, which are connected to preganglionic neurons projected from the sweat nucleus of the hypothalamus [77]. The skin conductance algesimeter measures micro-fluctuations in skin conductance in peaks per second (PPS) from a delivered micro-current in the palmar and plantar areas. The skin conductance increases transiently before the sweat evaporates, decreases again with sweat, and the consequent fluctuation is observed. Skin conductance is commonly measured in the hands for adults and in the feet for neonates. According to the manufacturers, the PPS parameter should be interpreted using the visual analogue scale (VAS) as follows: PPS within 0–0.07, no pain; PPI within 0.13–0.21, no pain or VAS less than 40; PPS to 0.26, patient is active and VAS around 40–50; PPS to 0.33, patient probably in pain with VAS around 60–80; and PPS within 0.40–0.7, patient probably in pain with VAS within 80–100.
Perioperative correlations to nociception stimuli have been reported [78,79], while skin conductance, measured as PPS, has shown moderate sensitivity and specificity at identified time points, with moderate to severe pain defined based on hormone plasma levels [70].
Skin conductance, however, does not reliably predict changes in stress hormone plasma levels during the intra-operative period [70]. Clinically relevant benefits of using skin conductance are unclear, which might rely on the nature of the biosignals, potential confounding effects, or the selected characterization used to describe this biosignal (i.e., PPS). According to the manufacturer, from a physiological perspective, the advantages of skin conductance monitoring are as follows: it is unaffected by temperature (22–42°C), general hypoxia, low or high blood volume, beta-blockers, or epinephrine, among other factors [68,78]. However, further research is needed to confirm these claims.
Spinal reflex-based monitoring
Nociceptive flexor reflex monitoring
The NFR, also known as the RIII reflex, system (Dolosys GmbH, Germany) describes the threshold electrical intensity required to elicit a spinal polysynaptic withdrawal reflex quantified by changes in electromyographic activity as a proxy of the analgesia level [80]. The electrical stimulus is applied to the sural nerve, and its effect is measured using biceps femoris muscle EMG. The amount of current required increases with analgesia [81–83]. The RIII reflex has also been used in studies of central sensitization and chronic pain [84]. Under propofol/remifentanil GA, the RIII threshold increases with remifentanil [82], with a higher predictive power of movement response to noxious stimulus (such as laryngeal mask airway insertion and skin incision) than other indices, such as the BIS, noxious stimulation response index, or composite variability index [83].
The NFR depends on sex, age, weight (obesity), and distinct physiologic factors [80]. Its limitations include the degree of neuromuscular blockade, skin impedance, peripheral nerve alterations, and muscular diseases.
Discussion
Intraoperative prediction of pain in the post-anesthesia care unit
Recently, several studies have aimed to evaluate the performance of different nociception monitoring index values at a single time during the intraoperative period (mainly during arousal before extubation) to predict postoperative pain upon arrival to the post-anesthesia care unit (PACU), using pain scores such as a 0-10 numerical rating scale (NRS) around 5–10 min after extubation. These research studies were not included in the individual descriptions of each nociception index of this review due to the contradictory results and the ground arguments against such a research approach.
For example, Boselli et al. [60], in their study on inhaled GA with remifentanil, reported excellent predictions of pain within 10 min of arrival in PACU from a single ANI measurement before extubation, with 86% sensitivity and 92% specificity to discriminate between patients with an NRS ≤ 3 and those with an NRS > 3. However, very different results were reported under sevoflurane-fentanyl anesthesia, when comparable single pre-extubation ANI measurements did not reflect different states of acute postoperative pain using the same NRS scale at 5 min intervals post-intubation in the PACU [85]. Similarly, pre-extubation SPI values (SPI > 30) were reported to predict postoperative NRS scores with a sensitivity and specificity of 50% and 89.7%, respectively [86]. However, in another study, despite the best SPI values for sensitivity/specificity to predict moderate-to-severe pain in the PACU (SPI values around 30), its predictive accuracy was poorer overall [23].
Furthermore, the severity of postoperative pain significantly influences skin conductance. Using cutoff values, the PPS may prove to be a useful tool for pain assessment in the postoperative period [87,88]. However, it is difficult to link such predictions when skin conductance does not reliably predict changes, for instance, in stress hormone plasma levels, throughout the intraoperative period [70]. There is a contradiction between index-insensitive behavior for short-term predictions versus longer-term predictions.
The possibility of single-value postoperative pain predictions, with a longer prediction horizon, has been recently reported [89], where only low NOL index values after skin incision significantly excluded moderate-severe pain in the PACU, with a negative predictive value of 83%, while other intraoperative NOL values, including at the end of the surgery, showed no significant prediction. This result conceptually invalidates all post-incision NOL estimations, with low sensitivity to detect a potential subjacent problem from an incision event that emerges later in the PACU. This result contradicts the forecasting principle, where the more in advance the forecasting, the more uncertainty.
Independent of the research type (surgery, anesthesia, nociception index, etc.), the expectation to predict pain assessments in the PACU from a single perioperative value seems unrealistic. Some of the main arguments are as follows:
Monitoring reflects only time-local conditions
Any monitoring system aims to continuously estimate the state of the system (patient) and track its changes. In fact, while maintaining the estimation power, the faster the better. If the system state varies, the monitor must reflect such variations, replacing previous single estimations. The validity of single estimations lasts as long as the system remains unchanged and the transitions the need for newer updates. Although the patient state transition from the intraoperative period to the PACU is short in time, it is very large in magnitude, invalidating pre-PACU single short-term estimations to predict PACU state.
Lack of trends
Based on the previous argument, any statistical forecasting method requires a minimum number of consecutive measurements (at least a few historical samples) to pick up some sort of trend for short-term prediction. For longer-term predictions and more complex systems and transitions, the historical data needs to be larger [90]. In general, the longer the prediction horizon, the larger the required information.
Lack of concomitant factor analysis
The patient’s pain perception in the PACU might depend on multiple factors, including patient demographic and historical data and surgery type and duration. Factors not included in the statistical analysis of the mentioned papers, as well as a lack of control groups and statistical post-hoc techniques for better noise level assessments may affect pain perception.
Conclusions
The latest development of better monitoring technologies for different aspects of the patient’s state under anesthesia has led to novel methods that focus on monitoring nociception. Nociception monitors can be based on ANS or CNS parameters; however, CNS-based methods focus on the cortex and subcortex of the brain, which is the target organ for analgesics. Therefore, in the future, CNS monitoring is likely to be the most prevalent method for monitoring analgesia and nociception during GA.
Notes
Funding
None.
Conflicts of Interest
P.M.V. is a research consultant for Quantium Medical and E.W.J. is the co-owner and CEO of Quantium Medical and works at Fresenius Kabi.
Author Contributions
Pablo Martinez-Vazquez (Conceptualization; Writing – original draft; Writing – review & editing)
Erik Weber Jensen (Supervision; Writing – review & editing)
