Edaravone attenuates sustained pial arteriolar vasoconstriction independently of endothelial function after unclamping of the abdominal aorta in rabbits
Article information
Abstract
Background
Cerebral blood flow (CBF) has direct effects on neuronal function and neurocognitive disorders. Oxidative stress from abdominal aortic surgery is important in the pathophysiology of CBF impairment. We investigated the effect of edaravone on the pial arteriolar diameter changes induced by abdominal aortic surgery and the involvement of the endothelium in the changes.
Methods
The closed cranial window technique was used in rabbits to measure changes in pial arteriolar diameter after the unclamping of abdominal aortic cross-clamping with an intravenous free radical scavenger, edaravone (control group [n = 6], edaravone 10 μg/kg/ min [n = 6], 100 μg/kg/min [n = 6]). Pial vasodilatory responses to topical application of acetylcholine (ACh) into the cranial window were investigated before abdominal aortic cross-clamping and after unclamping with intravenous administration of edaravone (control group [n = 6], edaravone 100 μg/kg/min [n = 6]).
Results
Aortic unclamping-induced vasoconstriction was significantly attenuated by continuous infusion of edaravone at 100 μg/kg/min. Topical ACh after unclamping did not produce any changes in pial arteriolar responses in comparison to before aortic cross-clamping in the control or edaravone groups. The changes in the response to topical ACh after unclamping in the saline and edaravone groups did not differ significantly.
Conclusions
Free radicals during abdominal aortic surgery might have contracted cerebral blood vessels independently of endothelial function in rabbits. Suppression of free radicals attenuated the sustained pial arteriolar vasoconstriction after aortic unclamping. Thus, the free radical scavenger might have some brain protective effect that maintains CBF independently of endothelial function.
Introduction
Aortic cross-clamping during open aneurysmectomy elevates systemic blood pressure and decreases cardiac output [1]. Aortic unclamping decreases systemic blood pressure and cardiac output and increases pulmonary arterial pressure [1]. Such hemodynamic instability has the potential to affect cerebral circulation and induce neurological complications [2–5]. The incidence of delirium after elective abdominal aortic aneurysm (AAA) repair is reported as 30–54% [6–8]. Postoperative cognitive dysfunction (POCD) occurs in 0–62% of patients undergoing abdominal aortic surgery [7,9]. Advanced age [3,5,9–12], baseline neurocognitive function [3–6,9,10,12], smoking [4–6,9], and open aortic repair [3–5] have been identified as risk factors for both delirium and cognitive dysfunction after abdominal aortic surgery.
Acute delirium has been associated with a significant reduction in regional and global cerebral blood flow (CBF) [13]. Early POCD after major noncardiac surgery is secondarily associated with impaired intraoperative CBF autoregulation in the elderly [14]. Maintenance of adequate CBF and cerebral metabolism is vital for the preservation of cognitive function [2,15]. We previously reported that abdominal aortic unclamping induced transient dilation of pial arterioles followed by sustained constriction, as assessed using rabbits [16–18]. CBF changes induced by abdominal aortic unclamping may be involved in postoperative neurological complications.
In addition to changes in CBF, oxidative stress may also be involved in perioperative neurocognitive disorders [19]. Under ischemic conditions, the metabolism of adenosine triphosphate generates oxygen-derived free radicals [1,20]. Oxygen free radical production increases from the completion of proximal anastomosis, reaches a maximum at 5 min after reperfusion, then gradually decreases [21]. Free radicals mediate tissue injury following post-ischemic reperfusion [22]. Peripheral oxidative stress may provoke microcirculatory dysfunction and compromise cerebral perfusion via dysregulation of blood-brain barrier integrity and activation of the endothelium, ultimately leading to the development of POCD [23]. Localized oxidative stress and reactive oxygen species have a strong impact on endothelial dysfunction, which disturbs proper perfusion and CBF [24].
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), which is known to be a free radical scavenger, is used in the treatment of acute stroke [25]. Edaravone inhibits lipid peroxidation by scavenging free radicals and exerts neuroprotective effects by inhibiting oxidative damage to the cerebrovascular endothelium and brain cells [26,27]. Pretreatment with edaravone significantly reduces the incidence of cognitive impairment after carotid endarterectomy [28]. Edaravone may also decrease the expression of pro-inflammatory cytokines, alleviate surgery-induced neuroinflammation, and reduce disruption of the blood-brain barrier [29].
We hypothesized that free radical scavenging would improve CBF changes after the unclamping of aortic cross-clamping. The objective of the present study was to investigate, using rabbits fitted with a cranial window, the effects of edaravone on the pial arteriolar diameter changes induced by abdominal aortic clamping. We also investigated the involvement of the endothelium in the cerebral arteriolar response caused by abdominal aortic unclamping.
Materials and Methods
The experimental protocols were approved by the Institutional Committee for Animal Care of Gifu University Graduate School of Medicine (Protocol No. 20-84). The experiments were performed using 30 anesthetized rabbits weighing 2.0–2.2 kg. Each animal was initially anesthetized with pentobarbital sodium (25 mg/kg intravenously) and maintained by inhalation of 0.5% isoflurane. Mechanical ventilation was administered through a tracheotomy tube using oxygen-enriched room air to maintain an inspiratory oxygen concentration of approximately 50%. The tidal volume and respiratory rate were adjusted to maintain the end-tidal carbon dioxide tension (PETCO2) at 35–40 mmHg, with PETCO2 monitored throughout the experiment. Polyvinyl chloride catheters were placed in the femoral vein for the administration of fluid (lactated Ringer’s solution: 5 ml/kg/h) and in the right axillary and left femoral arteries for the continuous monitoring of proximal and distal aortic pressures (PrAP and DiAP) and heart rate (HR), and for blood sampling (from the right axillary artery). The rectal temperature was maintained at 38.5–39.5°C with a heating blanket and a warming lamp. An incision was made in the skin of the lateral abdomen. The aorta was then taped just distal to the renal arteries for clamping.
A closed cranial window was used to observe the cerebral pial microcirculation. Each animal was placed in the sphinx posture, the scalp was retracted, and a 10-mm-diameter hole was created in the parietal bone. The dura and arachnoid membranes were opened carefully, and a ring with a glass coverslip was placed over the hole and secured using dental acrylic. The space under the window was filled with artificial cerebrospinal fluid (aCSF), the components of which were Na+ 157 mEq/L, K+ 3 mEq/L, Ca2+ 3 mEq/L, Mg2+ 1.3 mEq/L, Cl- 139 mEq/L, HCO3- 25 mEq/L, urea 40 mg/dl, and glucose 67 mg/dl (pH was adjusted to 7.48). This solution was freshly prepared 4 h before use and bubbled with 5% CO2 in air at 39.0°C for 15 min just before use. Four catheters were inserted into the ring. One was attached to a reservoir bottle containing aCSF to maintain a constant pressure of 5 mmHg in the window; the second was used to monitor the pressure in the window; the third was for the administration of experimental drugs and aCSF; and the fourth for draining the fluid. The temperature within the window was monitored using a thermometer (model 6510; Mallinckrodt Medical, USA) and was kept at 38.5–39.5°C.
Experiment 1
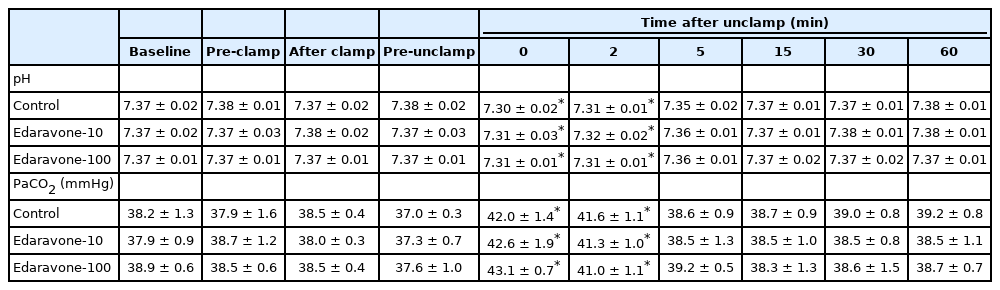
Rabbits (n = 18) were assigned to one of three groups as follows; systemic saline administration group (control group, n = 6) or systemic edaravone administration groups (E10 group, 10 μg/kg/min, n = 6; E100 group, 100 μg/kg/min, n = 6). All experiments were performed after at least 30 min of recovery from the surgical preparation. After each baseline pial arteriolar diameter measurement was made, each rabbit was administered saline (control group) or edaravone (E10 or E100 group) by intravenous infusion. All infusions continued throughout the experiment. The total doses of edaravone in the E10 and E100 groups were 2 mg and 20 mg, respectively. Fifteen minutes after the start of infusion, aortic clamping was performed for 20 min. The clamping and unclamping were performed gradually (each taking approximately 30 s) to minimize systemic hemodynamic changes. Measurements of two large (75–130 μm) and two small (40–75 μm) cerebral pial arteriolar diameters, hemodynamic variables (PrAP, DiAP, and HR), and various physiological variables (rectal temperature, intra-window temperature, arterial blood gas tensions, electrolytes, blood glucose, and blood pH) were performed at the following time points: immediately before the start of infusion, just before aortic clamping (pre-clamp), immediately after aortic clamping (after clamp), 20 min after clamping (before unclamping), and at 0, 2, 5, 15, 30, and 60 min after unclamping. The time point “0 min after unclamping” was actually 30 s after the start of unclamping, which took approximately 30 s to perform.
Experiment 2
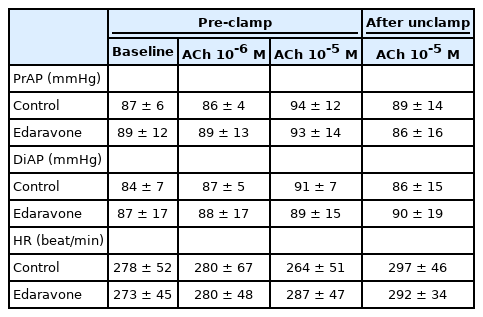
Rabbits (n = 12) were assigned to one of two groups; systemic saline administration group (control group, n = 6) or systemic edaravone administration group (100 μg/kg/min, n = 6). After each baseline pial arteriolar diameter measurement was made, each rabbit was administered saline or edaravone by intravenous infusion. All infusions continued throughout the experiment. The total dose of edaravone was 33 mg. Fifteen minutes after the start of the infusion, 10-6 M acetylcholine (ACh) was infused under the window for 5 min at a rate of 0.5 ml/min. To reestablish the baseline vessel diameters, the window was continuously flushed with aCSF at 0.25 ml/min for 25 min. Then, 10-5 M ACh was topically administered into the window for 5 min at a rate of 0.5 ml/min, and the window was continuously flushed with aCSF at 0.25 ml/min for 25 min. Aortic clamping was performed for 20 min. Clamping and unclamping were performed gradually (each taking approximately 30 s to perform). At 60 min after unclamping, 10-5 M ACh was topically administered into the cranial window at 0.25 ml/min for 5 min. Measurements of two cerebral pial arteriolar (35–160 μm) diameters, hemodynamic variables (PrAP, DiAP, and HR), and physiological variables (rectal temperature, intra-window temperature, arterial blood gas tensions, electrolytes, blood glucose, and blood pH) were taken at the following time points: immediately before the start of topical administration, after the topical administration of ACh (10-6 or 10-5 M) for 5 min, at 60 min after unclamping, and at 5 min after the second administration of 10-5 M ACh.
The pial arterioles were measured in each cranial window using a videomicrometer (Model VM-20; Flovel, Japan) on a television monitor, which received pictures from a microscope (Model SHZ-10; Olympus, Japan). The data from the pial views were stored on videotape for subsequent playback and analysis. The percentage changes recorded for individual arteriolar segments were averaged for each type (large or small) of vessel in each rabbit, and this average value was used in the statistical analysis.
Statistical analysis
In experiment 1, all variables used to assess the time-dependent effects within groups were tested using one-way analysis of variance (ANOVA) for repeated measurements, followed by the Tukey-Kramer test for post hoc comparisons. The differences between groups were examined using two-way ANOVA followed by one-way ANOVA for factorial measurements and an unpaired t-test with Bonferroni correction. In experiment 2, all variables used to assess the dose-dependent effects of ACh and the effects of abdominal aortic clamping within groups were tested using one-way ANOVA for repeated measurements followed by the Tukey-Kramer test for post hoc comparisons. Differences between groups were examined using an unpaired t-test. Statistical significance was set at P < 0.05. All values are presented as the mean ± standard deviation (SD).
Results
The effect of edaravone on the pial arteriolar diameter changes induced by aortic clamping and unclamping
In experiment 1, there were no significant differences in baseline hemodynamic or physiological variables between the three groups. Rectal and intra-window temperatures did not vary throughout the experiments in any group. PaO2, Na+, K+, and blood glucose levels were stable at all stages of the experiment in all groups (data not shown). In all groups, PrAP decreased significantly at 0 min after unclamping, and the DiAP value after clamping was significantly decreased compared to the baseline value. Both PrAP and DiAP recovered after unclamping. However, HR did not show significant changes in any group during the experiment (Table 1). In all groups, the arterial pH decreased significantly at 0 and 2 min after unclamping, and PaCO2 significantly increased at 0 and 2 min after unclamping (Table 2).
There were no significant differences between the groups in the baseline diameters of the large and small arterioles. For large pial arterioles, the diameters in all groups showed significant increases just after unclamping (control, 5.6% ± 2.8%, P = 0.041; E10, 6.7% ± 2.0%, P = 0.005; and E100, 6.4% ± 1.3%, P < 0.001). Diameters then decreased significantly, starting at 5 min after unclamping in the control group (P = 0.023), and at 15 min after unclamping in the E10 (P = 0.032) and E100 (P = 0.007) groups. The decreases in diameter remained significant (and, indeed, appeared to still be constricted) at 60 min after unclamping (control, -17.1% ± 4.3%, P < 0.001; E10, -13.2% ± 2.9%, P < 0.001; and E100, -7.9% ± 1.8%, P < 0.001). In the E100 group, but not in the E10 group, the decrease in diameter was significantly smaller than in the control group (15 min, P = 0.024; 30 min, P = 0.015; and 60 min, P < 0.001) (Fig. 1). For the small pial arterioles, the diameters in all groups showed significant increases at 0 and 2 min after unclamping (maximum increase: control, 10.0% ± 3.9%, P = 0.024; E10, 8.4% ± 3.7%, P= 0.001; and E100, 11.6% ± 1.7%, P < 0.001). Diameters then decreased significantly, starting at 15 min after unclamping in all groups (control, P < 0.001; E10, P = 0.001; and E100, P = 0.041). The decreases in diameter remained significant (and, indeed, appeared to still be constricted) at 60 min after unclamping (control, -27.3% ± 6.8%, P < 0.001; E10, -20.9% ± 2.4%, P < 0.001; and E100, -10.0% ± 4.8%, P < 0.001). In the E100 group, but not in the E10 group, the decrease in diameter was significantly smaller than in the control group (15 min, P = 0.018; 30 min, P = 0.005; and 60 min, P < 0.001) (Fig. 2).
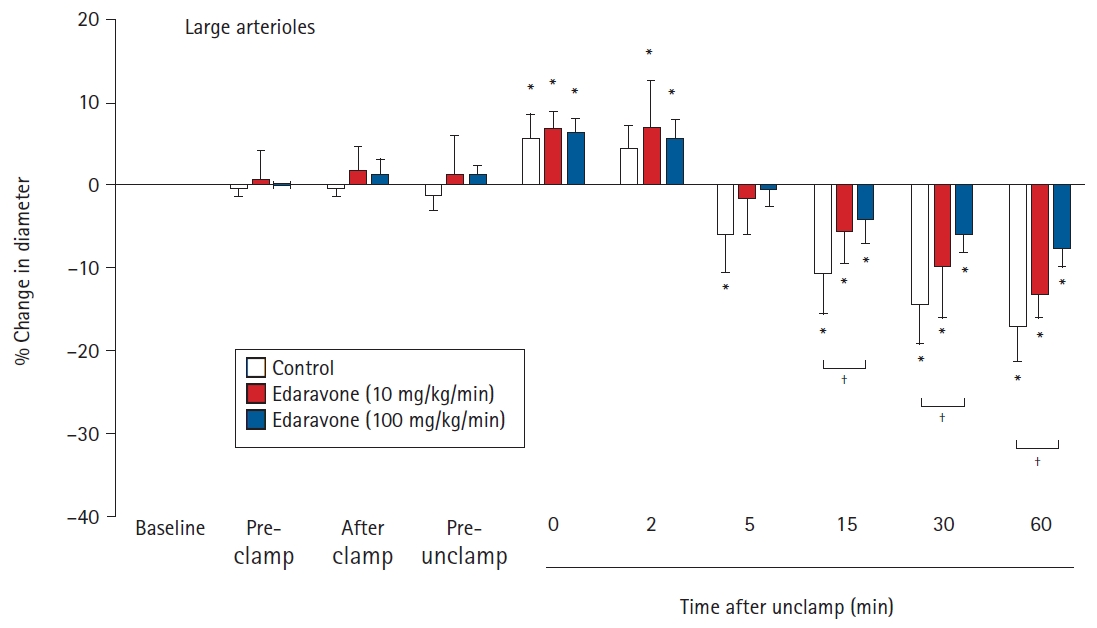
Effects of the continuous intravenous infusion of edaravone on the diameter of large cerebral pial arterioles (≥ 75 μm) to aortic clamping and unclamping in 18 rabbits. Data are expressed as the percentage change from the baseline diameter measured just prior to the administration of saline or edaravone. Data are shown for pre-clamp (15 min after administration), after clamp (immediately after clamping), pre-unclamp (20 min after clamping), and at 0, 2, 5, 15, 30, and 60 min after unclamping. Values are presented as mean ± SD. *P < 0.05 in comparison to baseline in the same group. †P < 0.05 in the comparison of the indicated values.
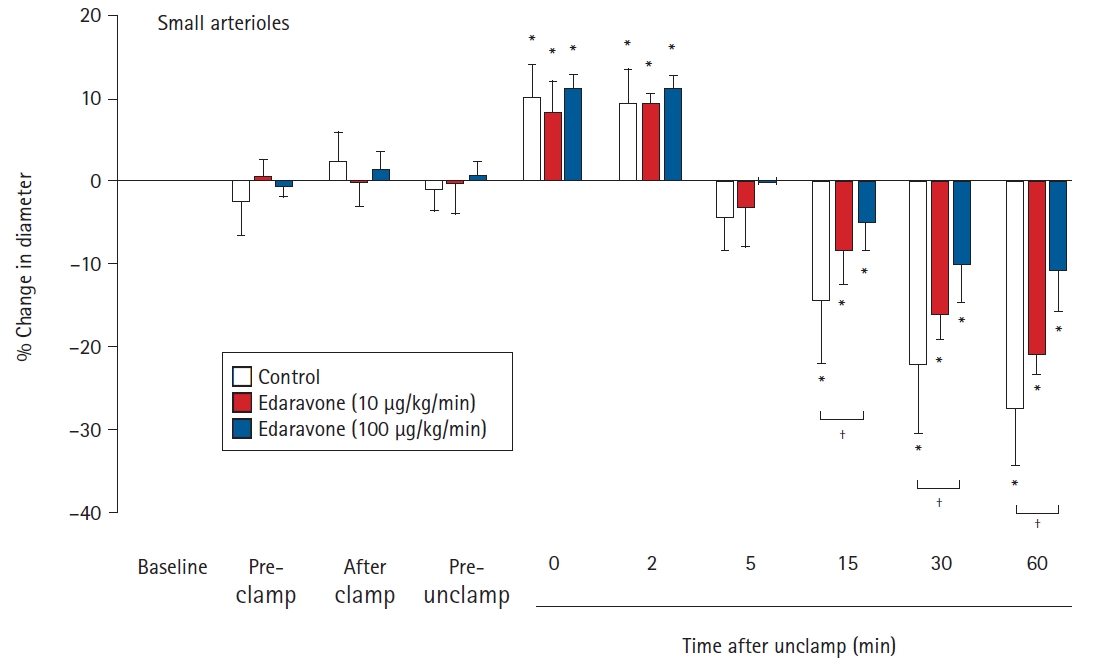
Effects of the continuous intravenous infusion of edaravone on the diameter of small cerebral pial arterioles (< 75 μm) to aortic clamping and unclamping in 18 rabbits. Data are expressed as the percentage change from the baseline diameter measured just prior to administration of saline or edaravone. Data are shown for pre-clamp (15 min after administration), after clamp (immediately after clamping), pre-unclamp (20 min after clamping), and at 0, 2, 5, 15, 30, and 60 min after unclamping. Values are presented as mean ± SD. *P < 0.05 in comparison to baseline in the same group. †P < 0.05 in the comparison of the indicated values.
The effect of topical ACh administration on the edaravone-induced diameter changes in the pial arterioles
In the first experiment, edaravone suppressed the decreases in vessel diameter after unclamping in both the large and small arterioles. Next, we investigated whether vascular endothelial function was involved in the edaravone-induced attenuation of the decrease in pial arteriolar diameters after unclamping. In the second experiment, we investigated the average percentage change in the diameters of large and small pial arterioles. We initially examined the arteriolar response to ACh (an endothelium-dependent vasodilator) administered through the cranial window before aortic clamping. There were no significant differences in the hemodynamics at baseline or after the topical administration of ACh in either group (Table 3). As in the first experiment, the cerebral pial arterioles dilated transiently just after unclamping and then constricted gradually up to 60 min after unclamping. All percentages represent changes in diameter with respect to the diameter immediately before the topical administration of ACh. In the control group, the cerebral pial arteriolar diameters were not significantly changed by the topical administration of 10-6 M ACh (3.6% ± 2.1%, P = 0.056). However, the first topical administration of 10-5 M ACh before clamping and the second topical administration of 10-5 M ACh at 60 min after unclamping led to significant increases in vessel diameter in comparison to that immediately before each ACh administration (8.7% ± 4.3% and 6.2% ± 2.1%, respectively, P < 0.001) (Fig. 3). The changes observed after the topical administration of 10-5 M ACh before clamping and at 60 min after unclamping did not differ significantly. In the edaravone group, as in the control group, the topical administration of 10-5 M ACh before clamping and at 60 min after unclamping significantly increased the diameter of the pial arterioles immediately before each ACh administration (before clamping, 8.1% ± 5.7%, P = 0.001; 60 min after unclamping, 8.8% ± 4.7%, P = 0.003) (Fig. 3). The changes observed after the topical administration of 10-5 M ACh before clamping and at 60 min after unclamping did not differ significantly (control, P = 0.266; edaravone, P = 0.602). There were no significant differences between the control and edaravone groups in the changes in pial arteriolar diameters after the second administration of 10-5 M ACh at 60 min after unclamping (P = 0.227) (Fig. 3).
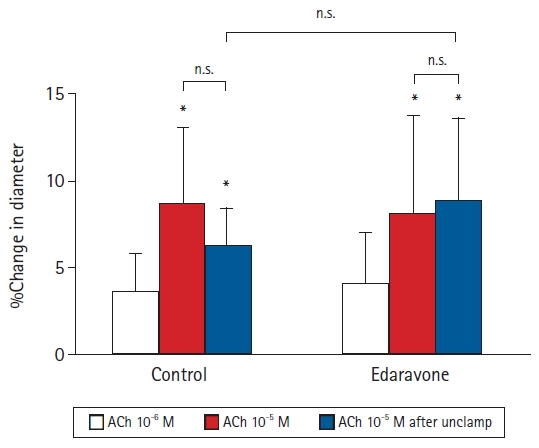
Effects of topically applied acetylcholine (ACh) on the diameter of pial arterioles before and after abdominal aortic clamping in 12 rabbits. Data are expressed as the percentage change from the baseline diameter measured just prior to administration of ACh. Values are presented as mean ± SD. *P < 0.05 in comparison to the diameters before ACh administration. n.s.: not significant.
Discussion
The present study showed that edaravone, a free radical scavenger, attenuated the sustained pial arteriolar constriction observed after the unclamping of an abdominal aortic cross-clamp. This result suggests that the scavenging of free radicals attenuates the vasoconstriction of pial arterioles. However, the vascular reactivity to topical ACh did not show any changes before aortic clamping and after unclamping, and the intravenous administration of edaravone did not induce any significant changes in the cerebrovascular reactivity to ACh. The vascular endothelium may not be involved in the attenuation of vascular constriction by edaravone.
Open abdominal aortic surgery with aortic cross-clamping is a risk factor for brain dysfunction such as delirium, stroke, and POCD [3,6,7]. A previous study using xenon CT reported a significant reduction in regional and global CBF during acute delirium [13]. Early POCD after major noncardiac surgery in the elderly is secondarily associated with impaired intraoperative CBF autoregulation [14]. We previously showed that sustained pial arteriolar vasoconstriction is caused by unclamping after aortic cross-clamping in rabbits [16-18]. Pial arteriolar vasoconstriction after unclamping may be involved in functional brain disorders. In the present study, edaravone attenuated aortic unclamping-induced pial arteriolar constriction. Based on our findings, it is possible that scavenging free radicals improves functional brain disorders by inhibiting cerebral vasoconstriction.
Reperfusion after aortic unclamping generates free radicals, reactive oxygen species, and cytokines in the lower limbs and the gastrointestinal tract [1,20]. These mediators can cause high-grade systemic oxidative stress and increased inflammation during open repair of AAA [21,22,30,31]. At the vascular level, the alterations in intracellular signaling induced by oxygen free radicals lead to endothelial dysfunction, reduced vasodilation, increased vascular contraction, and structural remodeling, leading to increased peripheral resistance and elevated blood pressure [32,33].
ACh stimulates the release of nitric oxide from vascular endothelium, thereby relaxing vascular smooth muscle cells and causing vasodilation [34]. In the present study, we confirmed that topical administration of ACh dilated cerebral pial arterioles in a dose-dependent manner. Since there were no significant differences in the cerebral pial arteriolar response to ACh before abdominal aortic clamping and after unclamping, it is suggested that endothelial function does not play a major role in the vasoconstriction induced by unclamping. Thus, the suppression of free radicals on the vascular endothelium by edaravone is probably not responsible for the alteration in vascular response observed in the present study.
The mechanism by which edaravone attenuates vasoconstriction independent of endothelial function remains unclear. However, edaravone has been reported to show a clear and selective inhibitory effect against hydroxyl radical-induced endothelium-independent vascular contraction [35]. Our results are consistent with those findings. Aortic cross-clamping and unclamping produce compounds that include free radicals [20], thromboxane A2 [36,37], endothelin-1 [38,39], and tumor necrosis factor alpha [40,41], which are formed in ischemic tissues [1]. After unclamping, these humoral factors and mediators are washed out from the ischemic area to the systemic circulation, which can then damage the microcirculation in remote systems, including the central nervous system [42]. Several reports have demonstrated that free radical scavengers can inhibit the synthesis of these humoral factors [43–45]. Exogenous antioxidants, such as edaravone, might attenuate cerebral vasoconstriction by inhibiting the synthesis of humoral factors rather than through the protection of the vascular endothelium from free radicals. Further investigation is necessary to demonstrate the involvement of humoral factors.
Continuous edaravone infusion at 3-10 mg/kg/h (50-167 μg/kg/min) has been successfully used to obtain free radical scavenging in rodents [46-48]. Therefore, we investigated the effects of two doses (10 and 100 μg/kg/min) of edaravone in the present study.
In conclusion, free radicals during abdominal aortic surgery might cause the contraction of cerebral blood vessels independent of endothelial function in rabbits. The suppression of free radicals attenuated sustained pial arteriolar vasoconstriction after aortic unclamping. Thus, free radical scavengers might have brain protective effects to maintain CBF independent of endothelial function.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Tomohiro Michino (Data curation; Investigation; Writing - original draft)
Kumiko Tanabe (Data curation; Writing - review & editing)
Motoyasu Takenaka (Methodology; Investigation)
Shigeru Akamatsu (Writing - review & editing)
Masayoshi Uchida (Data curation; Investigation)
Mami Iida (Methodology; Investigation)
Hiroki Iida (Supervision; conception and design; Writing - review & editing)