Effect of single dose preoperative intravenous ibuprofen on postoperative pain and opioid consumption: a systematic review and meta-analysis
Article information
Abstract
Background
Ibuprofen, a well-known analgesic, is commonly used as a component of a multimodal analgesic approach for postoperative pain. This systematic review and meta-analysis aimed to investigate whether a single-dose preoperative intravenous ibuprofen can reduce postoperative pain and opioid consumption.
Methods
PubMed/MEDLINE, Embase, Cochrane Library (CENTRAL), and Web of Science databases were searched to identify relevant studies published up to May 2020. Randomized controlled trials comparing preoperative single-dose intravenous ibuprofen effect with the control group on postoperative pain and opioid consumption after surgery under general anesthesia were included.
Results
Six studies involving 366 participants were included. Single-dose administration of intravenous ibuprofen preoperatively significantly reduced postoperative pain score on a scale of 0-10 at 1 h (MD: -1.64, 95% CI [-2.56, -0.72], P < 0.001, I2 = 95%), at 4-6 h (MD: -1.17, 95% CI [-2.09, -0.26], P < 0.001, I2 = 94%), and 24 h (MD: -0.58, 95% CI [-0.99, -0.18], P < 0.001, I2 = 90%). Cumulative opioid consumption, presented as fentanyl equivalents, was also reduced significantly in the ibuprofen group compared to placebo group until postoperative 4-6 h (MD: -56.35 μg, 95% CI [-101.10, -11.60], P < 0.001, I2 = 91%) and 24 h (MD: -131.39 μg, 95% CI [-224.56, -38.21], P < 0.001, I2 = 95%).
Conclusions
Preoperative single-dose intravenous ibuprofen can reduce postoperative pain and opioid consumption until 24 h postoperatively. Considering the high heterogeneity and small number of studies included, care should be taken when generalizing these findings.
Introduction
Poorly controlled postoperative pain may negatively affect patients’ clinical outcomes, such as postoperative complications and rehabilitation [1,2]. Multimodal analgesia is strongly recommended for the effective management of postoperative pain rather than using opioids alone [3,4]. In addition, multimodal analgesia is one of the key components of the enhanced recovery after surgery protocol, which aims to achieve early recovery through diverse approaches. Through multimodal analgesia, patients experience fewer opioid-induced adverse effects, early recovery, and early discharge by reducing the perioperative use of opioids [5].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are an important part of multimodal regimens for postoperative analgesia [6]. In combination with opioids, NSAIDs reduce opioid consumption and opioid-related side effects, such as nausea and vomiting [7].
Ibuprofen is an NSAID that inhibits cyclooxygenase enzymes, which convert arachidonic acid to prostaglandin H2, a mediator of inflammation, pain, and fever. Among the widely used NSAIDs, ibuprofen is less likely to cause gastrointestinal adverse events and cardiovascular risk [8,9]. Ibuprofen is preferred in various types of surgeries and patient populations because of its safety profile.
Although ibuprofen has a long history of use as an oral analgesic, intravenous ibuprofen has been used in clinical practice for just over 10 years, since its approval by the United States Food and Drug Administration in 2009. In adults, it is recommended to administer 400–800 mg of intravenous ibuprofen every 6 h as necessary, with a maximum limit of 3,200 mg per day [10].
The usefulness of multiple doses of IV ibuprofen in conjunction with opioids has been reported in the perioperative setting [11,12]. However, limited data are available on its usefulness when administered preoperatively through the intravenous route. Recently, a single dose of preoperative intravenous ibuprofen was suggested as an intervention to enhance the effectiveness of postoperative analgesia; however, inconsistent results were presented [13-18].
We hypothesized that the preoperative single dose administration of intravenous ibuprofen to surgical patients reduces postoperative pain and subsequent analgesic requirements. The objective of this study was to determine the effect of preoperative single dose intravenous ibuprofen on the severity of postoperative pain and opioid consumption by meta-analysis of data from previous randomized controlled studies.
Materials and Methods
Study design
This meta-analysis followed the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA; Appendix 1). The study was registered in the ‘International Prospective Register of Systematic Reviews’ (PROSPERO; https://www.crd.york.ac.uk/PROSPERO, no. CRD42020166141).
Information sources and search strategy
Two authors (SK and KK) searched PubMed/MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and Web of Science databases. The search terms included variants of terms, such as ‘ibuprofen,’ ‘intravenous,’ ‘postoperative pain,’ ‘analgesia,’ ‘opioid,’ ‘fentanyl,’ ‘morphine,’ and ‘patient controlled analgesia,’ as well as Medical Subject Heading or Embase Subject Heading terms (Appendix 2).
There was no limitation on the year of publication, but we limited the search to randomized controlled trials conducted on humans. The language of the article was limited to English and Korean. The date of the last search was May 12, 2020.
Study selection and eligibility criteria
After searching the articles from the databases listed above, two authors (SK and KK) selected the studies independently. Selection consisted of the following three steps: the two authors first selected the articles based on the title and then the abstract, and for the remaining articles, the two authors reviewed the full text of each article for the final selection. In case of disagreement, the two authors discussed the final selection of the articles until an agreement was reached.
The inclusion criteria were as follows: (1) patients under general anesthesia; (2) ibuprofen was administered intravenously; (3) ibuprofen was administered preoperatively, which is defined as before surgical incision; (4) control group using placebo was reported with results; and (5) primary outcomes of original articles were postoperative pain scores or opioid consumption.
Studies were excluded based on the following criteria: (1) articles not written in English or Korean; (2) patients not under general anesthesia; (3) ibuprofen was not administered intravenously; (4) ibuprofen was administered after skin incision or multiple times; (5) did not include appropriate postoperative outcomes; (6) non-randomized clinical trials; (7) non-human studies; and (8) did not compare with the appropriate control group. We also excluded articles that were not available in full text.
Risk of bias in individual studies
Based on the ‘risk of bias’ of the Review Manager software (RevMan, version 5.3; The Cochrane Collaboration, UK), two authors (SK and KK) independently evaluated the quality of articles. A third author (SL) was included to resolve disagreements when needed. Seven categories were included to assess the risk of bias: random sequence generation and allocation concealment for detecting selection bias, blinding of participants for performance bias, blinding of outcome assessor for detection bias, incomplete outcome data for attrition bias, selective reporting for reporting bias, and other biases that were not covered by the above categories. We specified the adequacy of the sample size calculation as ‘other bias,’ the seventh category. The risk of bias was rated as ‘high,’ ‘low,’ or ‘unclear’ in each original article. The agreement of two independent raters regarding the risk of bias for the seven categories was evaluated using Cohen’s kappa. The authors interpreted the Cohen’s kappa values based on Cohen’s suggestions as follows: (1) below 0.00, no agreement; (2) 0.00-0.20, slight agreement; (3) 0.21-0.40, fair agreement; (4) 0.41-0.60, moderate agreement; (5) 0.61-0.80, substantial agreement; and (6) 0.81-1.00, almost perfect agreement.
Data collection process and extracted items
Two authors (SK and KK) extracted data from the articles and cross-checked the data to avoid missing any information or extracting incorrect information. The extracted information included patient age, study design, publication year, authors’ first name, type of surgery, timing and dosage of study drug, and measured outcomes. The measured outcomes were as follows: postoperative pain score, postoperative analgesic regimen, and analgesic consumption. Two authors (SK and KK) independently extracted the data from the text, tables, and graphs. Cohen’s kappa was used to assess the agreement between the two authors on the extracted data, and the values were interpreted in a manner similar to the risk of bias.
We extracted pain scores at postoperative 1, 4-6, and 24 h to reflect immediate, early, and late postoperative pain, respectively, for the analysis of pain scores. If the pain score was not measured at postoperative 6 h in the original articles, we used pain scores measured at postoperative 4 h as a replacement [13,15–17]. Cumulative opioid consumption up to postoperative 4-6 h and 24 h was included. We extracted data on cumulative opioid consumption at postoperative 6 h, and if data were not available at 6 h, we extracted data measured at postoperative 4 h as a replacement [16,17].
To analyze the intensity of postoperative pain, pain scores measured using the visual analog scale (VAS) or numerical rating scale (NRS) were extracted from each study. When the studies evaluated pain scores during movement and resting state simultaneously, we only used scores assessed in the resting state. If the studies used opioids other than fentanyl, we converted them into fentanyl equivalents [14,18].
Statistical analysis
Summary measures
Pain scores were extracted using the mean and standard deviation at specified time points. We also extracted the mean and standard deviation of cumulative opioid consumption in a similar manner.
Synthesis of results
The VAS and NRS scores were strongly correlated [19], and pain scores were measured on the same scale (0-10) in all included studies. In addition, opioid consumption was converted into fentanyl equivalents (μg). Therefore, we calculated the mean differences (MDs) for continuous outcomes (postoperative pain scores or cumulative opioid consumption). We calculated the 95% CI for all estimates. A random-effect model was used for all trial results, because of the possibility of different effect sizes across the studies. To measure heterogeneity among the trials, Higgins’ I2, the heterogeneity statistic Cochrane’s Q, and the corresponding P values were calculated. We considered I2 > 50% as significant heterogeneity.
Sensitivity analysis was performed by leave-one-out analyses using meta and dmetar packages in R software (version 3.6.3, R Foundation for Statistical Computing, Austria).
Publication bias was not assessed in this meta-analysis, because the number of included studies was less than 10.
We used Review Manager (RevMan, version 5.3, The Cochrane Collaboration) and R software (version 3.6.3, R Foundation for Statistical Computing, Austria) for all analyses.
Results
Study selection and characteristics
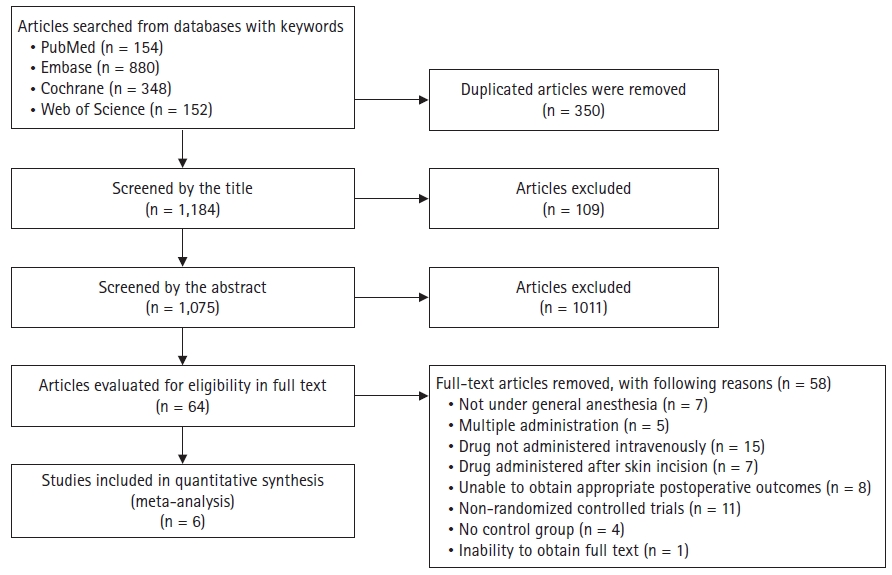
The authors obtained 1,534 articles after an initial database search of PubMed (n = 154), Embase (n = 880), the Cochrane Library (n = 348), and Web of Science (n = 152). We excluded 350 duplicate articles. Two authors reviewed the articles independently, and subsequently excluded 109 reports based on the title and 1011 articles based on the abstract. Final full-text reviews were performed on the remaining 64 articles. Of the 64 articles, 58 were excluded based on exclusion criteria. Details of the reason for the exclusion are described in Fig. 1.
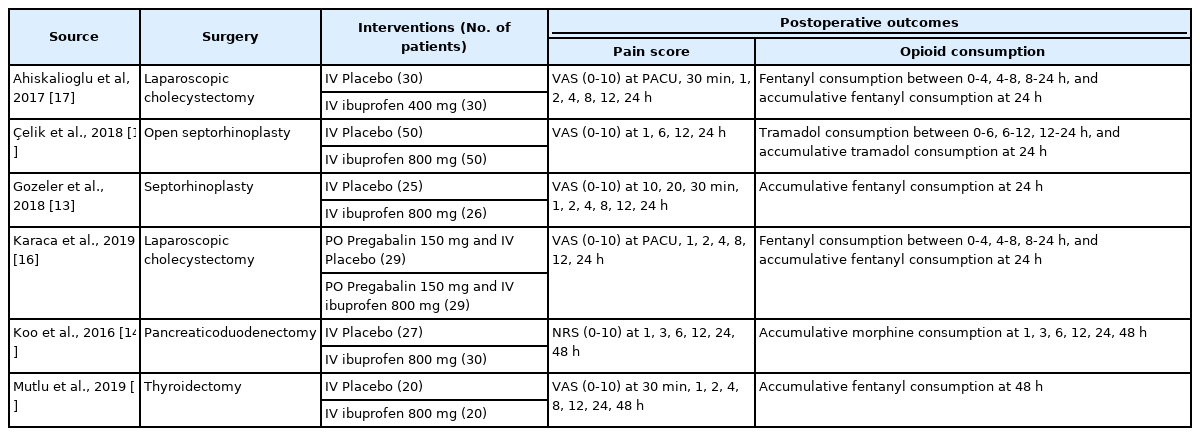
The characteristics of the six studies are presented in Table 1. All articles were randomized clinical trials. The types of surgeries were diverse, including laparoscopic cholecystectomy [16,17], septorhinoplasty [13,18], pancreaticoduodenectomy [14], and thyroidectomy [15]. Ibuprofen was administered intravenously in all the studies; however, the doses were different: one study administered 400 mg of ibuprofen [17], while other studies injected 800 mg of ibuprofen. Furthermore, opioids used in these studies were diverse and included fentanyl [13,15–17], morphine [14], and tramadol [18]. The timing of pain score measurement and opioid consumption varied among the studies.
Quality assessment of included studies (risk of bias within studies)
The risk of bias assessment indicated that all included studies had low bias (Figs. 2 and 3). All trials were evaluated as having a low risk of random sequence generation. Of all included studies, 66.6% in allocation concealment, 16.6% in blinding of participants and personnel, 16.6% in blinding of outcome assessment, 66.6% in selective reporting, and 16.6% in other bias categories were assessed as ‘unclear’. Only two trials [14,18] were clear about allocation concealment, while other trials (66.6%) did not describe the allocation concealment method in detail. With respect to blinding of patients, one study [18] did not mention the term ‘double-blinded’ or describe its method of blinding. Furthermore, it did not specify blinding of the outcome assessor method, so we assessed it as ‘unclear’. Only two studies [14,16] provided information regarding protocols written in advance. All studies were rated as ‘low’ in other bias, except for two trials: one study [15] without calculation of required sample size and power analysis rated as ‘unclear,’ and the other [18] with inappropriate power analysis rated as ‘high.’

Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
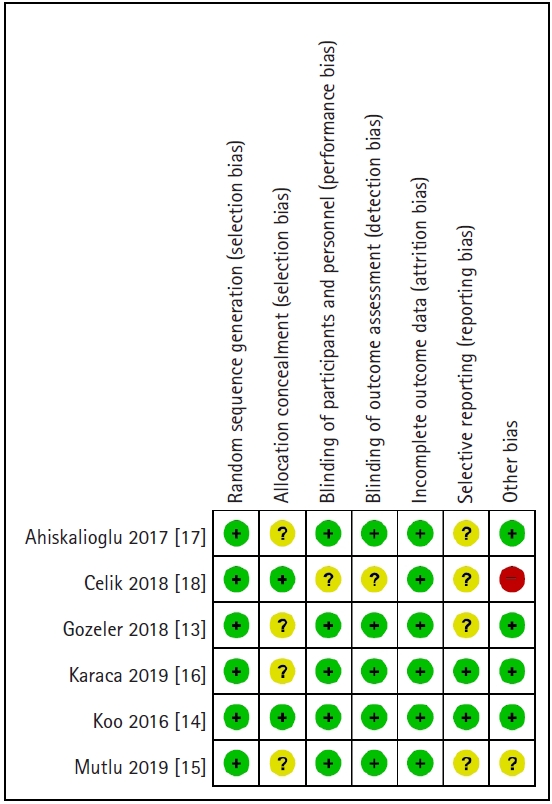
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. +: low risk, ?: unclear risk, -: high risk.
Agreement between the two raters for assessing the risk of bias was moderate (Cohen’s kappa = 0.52).
Meta-analysis
One study [14] reported the effects of intraoperative remifentanil infusion along with preoperative ibuprofen on postoperative pain and opioid consumption. Patients in this study received intraoperative remifentanil infusion targeting 4 ng/ml or 1 ng/ml as effect-site concentration, with or without preoperative ibuprofen administration. In this study, we extracted the data from subgroups with low remifentanil infusion only, because most of the other articles included in our meta-analysis did not use opioid infusion or used a low infusion rate of opioids during surgery.
Pain scores at postoperative 4 h were extracted from four reports [13,15–17], since data at 6 h were not available. Opioid consumption of one study [15] was not included in the analysis of opioid consumption owing to a lack of information, and in two studies [16,17], opioid consumption at postoperative 4 h was used because there were no data available at postoperative 6 h.
Agreement between the two raters for data extraction was substantial (Cohen’s kappa = 0.63).
Pain intensity at postoperative 1 h
A meta-analysis of six studies [13–18] (n = 366; 185 in the ibuprofen group and 181 in the control group) showed that pain scores measured at 1 h postoperatively were significantly reduced in the preoperative ibuprofen group (MD: -1.64, 95% CI [-2.56, -0.72], P < 0.001, I2 = 95%) (Fig. 4A).

Forest plot: Effect of ibuprofen on postoperative pain scores. (A) Postoperative 1 h. (B) Postoperative 4-6 h. (C) Postoperative 24 h. SD: standard deviation, IV: inverse variance.
In the sensitivity test, the pain score measured at 1 h postoperatively was lower (MD: -1.94, 95% CI [-2.30, -1.57], P < 0.001, I2 = 29%) than the estimated pooled effect with consistent direction and significance after excluding the outlier study [14]. It can be concluded that the results were robust. In addition, there was an effect of a reduction in heterogeneity to an acceptable level.
Pain intensity at postoperative 4-6 h
A meta-analysis of six studies [13–18] (n = 366; 185 in ibuprofen group and 181 in control group) showed that the preoperative ibuprofen group reported lower pain intensity with a statistical significance (MD: -1.17, 95% CI [-2.09, -0.26], P < 0.001, I2 = 94%) (Fig. 4B).
In the sensitivity analysis, the effects of pain intensity at 4-6 h were significantly reduced (MD: -0.79, 95% CI [-1.29, -0.30], P = 0.0016, I2 = 61%) compared to the pooled effect after excluding one trial [18], which was an outlier in this analysis. However, the results were reliable, because the direction and significance were maintained. Although the heterogeneity decreased, it was still of moderate intensity; hence, the results should be interpreted carefully.
Pain intensity at postoperative 24 h
A meta-analysis of data from six studies [13-18] (n = 366; 185 in the ibuprofen group and 181 in the control group) demonstrated that pain scores of the ibuprofen group were lower than those of the control group (MD: -0.58, 95% CI [-0.99, -0.18], P < 0.001, I2 = 90%) (Fig. 4C).
In the sensitivity analysis, the pain score of the ibuprofen group measured at 24 h postoperatively was still significantly more effective (MD: -0.75, 95% CI [-1.17, -0.32], P < 0.001, I2 = 88%) than the estimated effect after excluding one trial [14], an outlier. As the direction and significance of the results were maintained, the results were regarded as robust.
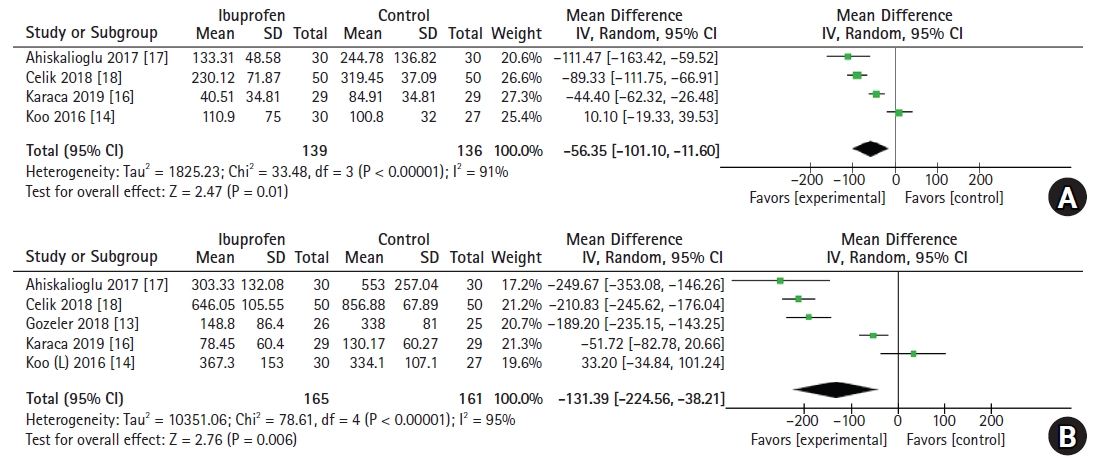
Accumulative opioid consumption at postoperative 4-6 h
A total of four studies [14,16–18] (n = 275; 139 in the ibuprofen group and 136 in the control group) showed data on the accumulative opioid consumption at postoperative 4-6 h. Preoperative ibuprofen administration significantly reduced opioid consumption, which was presented as fentanyl equivalents (MD: -56.35 μg, 95% CI [-101.10, -11.60], P < 0.001, I2 = 91%) (Fig. 5A). Sensitivity analysis did not show any outliers.
Accumulative opioid consumption at postoperative 24 h
Meta-analysis of five studies [13,14,16–18] (n = 326; 165 in the ibuprofen group and 161 in the control group) showed that cumulative opioid consumption at 24 h postoperatively was lower in the ibuprofen group (MD: -131.39 μg, 95% CI [-224.56, -38.21], P < 0.001, I2 = 95%) (Fig. 5B).
In the sensitivity analysis, the effect size of cumulative opioid consumption at 24 h increased (MD: -170.70 μg, 95% CI [-265.63, -75.76], P < 0.001, I2 = 95%) after excluding one trial [14], which was indicated to be an outlier.
Discussion
Recently, the multimodal analgesic approach has been in the spotlight as a way to reduce pain. Supplemental analgesics for postoperative pain can be administered before, during, or after surgery. Although some studies have shown the effect of preoperative drug administration on postoperative pain and opioid consumption [20,21], limited data are available regarding the preoperative administration of ibuprofen through the intravenous route. In this meta-analysis, the data showed statistically significant reductions in postoperative pain scores and opioid consumption when ibuprofen was administered intravenously before surgery.
In our study, postoperative pain scores were reduced at all analyzed time points (postoperative 1, 4-6, and 24 h) after a single dose of ibuprofen. The MDs in postoperative pain scores on a scale of 0-10 reduced over time: -1.64, -1.17, and -0.58, for postoperative 1, 4-6, and 24 h, respectively. Therefore, it can be inferred that the effect of preoperative single dose administration of ibuprofen decreases with time; however, it is still effective until postoperative 24 h. Accounting for the duration of surgery, postoperative 24 h is the time point at which more than 24 h have elapsed after ibuprofen administration. Considering that the half-life of intravenous ibuprofen is approximately 2 h [22] and the mean duration of ibuprofen is approximately 6-8 h, this result is interesting and suggests that the effect of ibuprofen persists even when the plasma concentration of ibuprofen is close to zero. In this regard, it is speculated that preoperative administration of ibuprofen may also have a preemptive effect. Preemptive analgesia limits pain response by suppressing initial pain sensitization [23].
We demonstrated that preoperative ibuprofen reduced postoperative opioid consumption and postoperative pain. This seems reasonable, as the severity of postoperative pain is one of the main factors determining analgesic requirements [24]. However, despite the reduction in the severity of pain, flurbiprofen, a similar NSAID, did not reduce opioid consumption in other meta-analyses [21].
Although we could not conduct subgroup analysis according to the dose of ibuprofen in this meta-analysis, it would be useful to compare whether the effect of ibuprofen varies depending on the dose (400 or 800 mg). In addition, a comparison between ibuprofen and other analgesics in various settings, including patient characteristics, type of surgery, and anesthetic techniques, may contribute to the determination of optimal multimodal analgesia.
Opioid-related side effects, such as postoperative nausea and vomiting, can be expected to be reduced, as opioid consumption was reduced when ibuprofen was administered preoperatively, as reported by another study using flurbiprofen [25]. In our meta-analysis, we could not analyze the effect of preoperative ibuprofen on postoperative nausea and vomiting, since the included articles reported outcomes differently. One article [13] reported the number of patients who experienced nausea and vomiting, while others [14–18] reported events of nausea and vomiting. In addition, some articles [13,14,16,17] counted nausea and vomiting as one criterion, while others [15,18] counted them as two different criteria. Therefore, we concluded that the outcomes of postoperative nausea and vomiting could not be synthesized appropriately for the meta-analysis.
In this meta-analysis, the heterogeneity in all study outcomes was quite high. Such high heterogeneity could be the result of various study designs, types of surgeries, anesthetic techniques, doses of anesthetic drugs, or drugs that were administered simultaneously, such as remifentanil or gabapentin [14,16]. The doses and type of rescue drugs and timing of rescue drug administration were also different between trials.
In this meta-analysis, heterogeneity was reduced after the exclusion of one study [18] from all analyses (results not shown). Unlike other studies, this study used tramadol to control postoperative pain. Tramadol targets opioid receptors and inhibits the reuptake of noradrenaline and serotonin [26]. The unique mechanisms of action of tramadol may have contributed to its high heterogeneity.
This study has some limitations. First, only a few studies were included. Although we did not limit the publication year when we selected the articles, only a few studies were included. This could be because there were few published articles on this topic, or the inclusion criteria of our meta-analysis were very strict and limited. Second, heterogeneity between studies was high in all the analyses of postoperative outcomes, possibly caused by several factors, such as different surgery types and different doses of study drugs or combination drugs. We attempted to conduct subgroup analysis to determine the cause of high heterogeneity; however, owing to the small number of included studies, we could not classify them into homogenous subgroups. Third, data and analysis of opioid consumption could have been more accurate if rescue analgesia was included in opioid consumption; however, this was not performed because of a lack of information. All studies recorded the number of patients who required rescue analgesia, and not the number of rescue analgesia administrations. Therefore, we were unable to calculate the exact amount of rescue analgesics injected.
In conclusion, preoperative single dose intravenous ibuprofen can reduce pain and opioid consumption until 24 h postoperatively. We expect that these findings can contribute to multimodal analgesia by increasing the efficiency of postoperative pain management. However, the analysis reported high heterogeneity within trials, probably owing to variations in study designs and small sample sizes. In addition, the type of surgery was limited in our study. Therefore, care should be taken when generalizing these findings. Further studies with similar designs are needed to increase the reliability of evidence and to determine the effect of preoperative administration of ibuprofen on postoperative pain intensity and opioid consumption.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Su Yeon Kim (Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft)
Sangseok Lee (Formal analysis; Validation; Writing – review & editing)
Yeji Lee (Writing – review & editing)
Hyunho Kim (Writing – review & editing)
Kye-Min Kim (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Writing – review & editing)
References
Appendices
Appendix 2. Search strategy for each database.
PubMed/MEDLINE
#1. ibuprofen
#2. intravenous* OR iv OR parenteral*
#3. pain, postoperative [MeSH Terms]
#4. postop* OR postsurg* OR postprocedur* OR “post op*” OR “post procedur*” OR “after surg*” OR “after op*” OR “after procedur*”
#5. pain OR analges* OR opioid* OR morphine OR fentanyl OR “patient controlled analgesia” OR pca
#6. #4 AND #5
#7. #3 OR #6
#8. randomized controlled trial [pt]
#9. controlled clinical trial [pt]
#10. randomized [tiab] OR randomized [tiab]
#11. placebo [tiab]
#12. drug therapy [sh]
#13. randomly [tiab]
#14. trial [tiab]
#15. groups [tiab]
#16. #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#17. animals [mh] NOT humans [mh]
#18. #16 NOT #17
#19. #1 AND #2 AND #7 AND #18
Embase
#1. ‘ibuprofen’/exp OR ibuprofen
#2. ‘intravenous drug administration’/exp OR ‘intravenous drug administration’ OR intravenous* OR iv OR parenteral*
#3. ‘postoperative pain’/exp OR ‘postoperative pain’
#4. Postop* OR postsurg* OR postprocedur* OR ‘post op*’ OR ‘post surg*’ OR ‘post procedur*’ OR ‘after surg*’ OR ‘after op*’ OR ‘after procedur*’
#5. ‘pain’/exp OR pain OR analges* OR opioid* OR ‘morphine’/exp OR morphine OR ‘fentanyl’/exp OR fentanyl OR ‘patient controlled analgesia’/exp OR ‘patient controlled analgesia’ OR ‘pca’/exp OR pca
#6. #4 AND #5
#7. #3 OR #6
#8. Random*:ab,ti OR ((clinical NEXT/1 trial*):de,ab,ti) OR placebo*:de,ab,ti OR ((double NEXT/1 blind*):ab,ti) OR group*
#9. #1 AND #2 AND #7 AND #8
The Cochrane Library
#1. (ibuprofen):ti,ab,kw (Word variations have been searched)
#2. (intravenous* OR iv OR parenteral*):ti,ab,kw (Word variations have been searched)
#3. MeSH descriptor: [Pain, Postoperative] explode all trees
#4. (postop* OR postsurg* OR postprocedur* OR “post op*” OR “post surg*” OR “post proceur*” OR “after surg*” OR “after op*” OR “after procedur*”):ti,ab,kw (Word variations have been searched)
#5. (pain OR analges* OR opoioid* OR morphine OR fentanyl OR “patient controlled analgesia” OR pca):ti,ab,kw (Word variations have been searched)
#6. #4 AND #5
#7. #3 OR #6
#8. #1 AND #2 AND #7 in Trials
Web of Science
#1. TS=(ibuprofen)
#2. TS=(iv OR intravenous* OR parenteral*)
#3. TS=(postop* OR postsurg* OR postprocedur* OR “post op*” OR “post surg*” OR “post procedur*” OR “after surg*” OR “after op*” OR “after procedur*”)
#4. TS=(pain OR analges* OR opioid* OR morphine OR fentanyl OR “patient controlled analgesia” OR pca)
#5. #3 AND #4
#6. TS=(random* OR “clinical NEXT/1 trial*” OR placebo* OR “double NEXT/1 blind*” OR group*)
#7. #1 AND #2 AND #5 AND #6