Hemodynamic effects of norepinephrine versus phenylephrine infusion for prophylaxis against spinal anesthesia-induced hypotension in the elderly population undergoing hip fracture surgery: a randomized controlled trial
Article information
Abstract
Background
Elderly population are at increased risk of spinal anesthesia-induced hypotension increasing their risk for postoperative morbidity and mortality. This study aimed to compare the hemodynamic effects of prophylactic infusion of norepinephrine (NE) versus phenylephrine (PE) in elderly patients undergoing hip fracture surgery under spinal anesthesia.
Methods
Elderly patients scheduled for hip fracture surgery were randomized to receive either NE infusion (8 µg/min) (NE group, n = 31) or PE infusion (100 µg/min) (PE group, n = 31) after spinal anesthesia. Outcomes included mean heart rate, mean blood pressure, cardiac output, incidence of spinal anesthesia-induced hypotension, incidence of bradycardia, and incidence of hypertension.
Results
Sixty-two patients with a mean age of 71 ± 6 years were included in the final analysis (31 patients in each group). The NE group showed a higher mean heart rate and cardiac output than the PE group. The NE group had a lower incidence of reactive bradycardia (10% vs. 36%, P = 0.031) and hypertension (3% vs. 36%, P = 0.003) than the PE group. No study participant developed hypotension, and the mean blood pressure was comparable between the two groups.
Conclusions
Both NE and PE infusions effectively prevented spinal anesthesia-induced hypotension in elderly patients undergoing hip fracture surgery. However, NE provided more hemodynamic stability than PE; maintaining the heart rate, higher cardiac output, less reactive bradycardia, and hypertension.
Introduction
In the elderly, the incidence of spinal anesthesia-induced hypotension can be as high as 73% [1], exposing this vulnerable group to reduced organ perfusion and increasing the risk of perioperative morbidity and mortality [2,3].
Sympathetic blockade after spinal anesthesia results in venodilation, which reduces venous return and hence cardiac output and results in hypotension [4]. Therefore, the use of alpha-adrenergic agonist drugs induces constriction of compliant veins, effectively reverses the vasodilatory effect of spinal anesthesia, and reduces the need for unnecessary fluid loading [5]. In the elderly population, the mechanism of hypotension differs from that in young adults, as it is mainly caused by a reduced stroke volume rather than systemic vascular resistance [6].
The prophylactic use of vasopressor agents for preventing spinal anesthesia-induced hypotension is warranted in high-risk populations and is currently recommended in obstetric anesthesia [7]. In the elderly population, although the use of vasopressor prophylaxis makes sense, the available data for drug groups and doses are sparse. Phenylephrine (PE) is a synthetic α-agonist vasoconstrictor that has been evaluated (in maintaining hemodynamic parameters) during spinal anesthesia in the elderly population [1]. However, PE (being a pure α agonist) was reported to decrease the heart rate and cardiac output [8], which limits its use in patients with reduced cardiac contractility; this fact makes the use of PE in elderly patients questionable. Furthermore, Jakobsson et al. [9] reported that spinal anesthesia-induced hypotension in the elderly could be a sign of impaired cardiac performance; therefore, PE might not be the ideal vasopressor for use in this population.
Norepinephrine (NE) has α agonistic and weak β agonistic activity; thus, in addition to its vasoconstriction properties, NE has modest cardio-stimulatory function compared to PE [10]. NE has been successfully evaluated for prophylaxis against spinal anesthesia-induced hypotension in obstetric anesthesia [8,11,12]. In this study, we aimed to compare the hemodynamic effect of prophylactic NE versus PE infusion in elderly patients undergoing hip fracture surgery under spinal anesthesia.
Materials and Methods
This randomized controlled trial was conducted in the orthopedic surgical theater at Cairo University Hospital from January to March 2020 after the Institution Research Ethics Committee approval (No: D-7-2019) and clinical trial registration (NCT04195321). Written informed consent was obtained from all patients before their enrollment in the study. The study was conducted in accordance with the Helsinki Declaration-2013.
Elderly patients (> 60 years), American Society of Anesthesiologists physical status I–II, scheduled for hip fracture surgery under spinal anesthesia, were included. Patients with known cardiac abnormalities (left ventricular ejection fraction < 50% or decompensated heart failure, heart block, arrhythmia), uncontrolled hypertension, hyperthyroidism, and monoamine oxidase inhibitor use were excluded from the study. Patients with a history of allergy to any of the study drugs and patients who had contraindications for spinal anesthesia were excluded.
Patients were randomly allocated at a 1 : 1 ratio to either the NE group or the PE group, using a computer-generated random sequence and concealed envelopes that contained the drug preparation instruction.
Preoperative fasting instructions were 6 hours for solid food, and clear fluid was allowed up to 2 hours preoperatively. Upon arrival to the operating room, routine monitors (electrocardiogram, pulse oximetry, and non-invasive blood pressure monitor) were applied; an intravenous line was secured, and routine pre-medications (ranitidine 50 mg slow IV) were administered. Baseline preoperative blood pressure was recorded in the supine position with an average of 3 readings, with a difference of less than 5 mmHg.
Before the initiation of spinal anesthesia for all study patients, an electrical cardiometry device ICONTM (Osypka Cordiotonic, German) was applied to the patient through 4 electrodes at the following sites: below the left ear, above the midpoint of the left clavicle, left mid-axillary line at the level of the xiphoid process, and 5 cm inferior to the third electrode. Stroke volume variability (SVV) was measured while the patient maintained standard calm breathing at 8 breaths/min for 1 min before the intrathecal injection. The patient with SVV ≥ 13% was considered a fluid responder [13] and received a fluid bolus of 8 ml/kg Ringer’s acetate (FIBCO, Egypt) over 10 min. The fluid bolus was repeated until the SVV became less than 13%, and spinal anesthesia was then performed.
After the induction of spinal anesthesia, maintenance fluid at 2 ml/kg/h of Ringer acetate was initiated.
Spinal anesthesia was performed in the sitting position at the level of L2–3 or L3–4 interspaces with a 25-gauge spinal needle. After confirming cerebrospinal fluid flow, 10 mg of 0.5% hyperbaric bupivacaine plus 25 µg fentanyl were injected. The degree of sensory block (cold test by alcohol gauze) was assessed in the study with the goal of T6–8 dermatomal level block. If spinal anesthesia failed, the patient was excluded from the study and was managed according to the attending anesthetist’s discretion, local expertise, and clinical practice.
Vasopressor infusion according to the randomization was initiated after obtaining cerebrospinal fluid, through the same line as IV fluids aided by a three-way stopcock, after the induction of spinal anesthesia.
Patients in the NE group received NE infusion (8 mg norepinephrine bitartrate/4 ml, equivalent to 4 mg norepinephrine base/4 ml, Alexandria Co., Egypt) at a starting rate of 1 ml/min of 8 µg/ml solution (prepared by diluting 4 mg NE in 500 ml saline).
Patients in the PE group received PE infusion (10 mg/1 ml ampoule, Sterop Co., Belgium) at a starting rate of 1 ml/min of 100 µg/ml solution (prepared by diluting 10 mg of PE in 100 ml saline).
All drug preparations were performed by a research assistant who was also responsible for opening the envelope and group assignment with no further involvement in the study. After diluting the drug as instructed, the diluted drug was then withdrawn into a 50 ml syringe to be given to the attending anesthetist.
If any episode of spinal anesthesia-induced hypotension occurred (defined as mean blood pressure < 80% of the baseline reading during 45 min after the spinal block), the protocol was set to be managed by 5 µg NE, and the infusion rate was increased by 20%. If the hypotensive episode persisted for 2 min, another bolus of norepinephrine was administered.
If bradycardia (defined as heart rate ≤ 50 beats/min) with hypotension occurred, it was managed with 0. 5 mg of atropine IV. If bradycardia occurred with hypertension (mean blood pressure > 125% of baseline), the vasopressor infusion was stopped.
Hypertension was defined as an increased mean blood pressure of > 125% of the baseline reading. Hypertensive episodes that persisted for 2 consecutive readings were managed by reducing the infusion by 50%. If hypertension persisted after the reduction of the infusion rate, the vasopressor infusion was stopped. The vasopressor resumed at 50% of the starting dose if there was a further decline in blood pressure.
Vasopressor infusion was continued for 45 min after spinal anesthesia. If the patient develops hypotension after discontinuation of the infusion, blood pressure management will depend on the fluid status of the patient. If hypotension occurred because of blood loss, 3 : 1 Ringer’s acetate was infused as a replacement until transfusion threshold is met; thereafter, the packed red blood cell is given with a target hemoglobin level of ≥ 9 g/dl. In case hypotension was unrelated to blood loss, the vasopressor was re-initiated at the last dose before discontinuation.
The primary outcome was mean heart rate during vasopressor infusion.
For secondary outcomes heart rate, cardiac output, and mean blood pressure (baseline, then every 2 min after spinal anesthesia for 20 min, then every 5 min until the end of the operation) were analyzed. Other outcomes included the incidence of bradycardia, tachycardia (heart rate >100 beats/min), spinal anesthesia-induced hypotension, and reactive hypertension. Pre- and intraoperative fluid volume, blood loss, and the need for blood transfusion were recorded.
Statistical analysis
The mean heart rate during the first 45 min after spinal anesthesia under PE infusion was 68 ± 9 beats/min, as calculated from a pilot study. We calculated our sample size to detect a mean difference of 10 beats/min between both groups. Using MedCalc Software version 14 (MedCalc Software bvba, Belgium), a minimum of 28 patients were required to have a study power of 80% and an alpha error of 0.05. We performed a more conservative calculation by increasing the study power to 99%, which increased the sample size to 62 patients (31 patients per group). To compensate for any dropouts, the final number of envelopes was 68 (34 per group).
Data analysis was performed using Statistical Package for Social Science (SPSS) software, version 21 for Microsoft Windows (SPSS Inc., USA). Categorical data are reported as numbers and percentages and were analyzed using the chi-square test. Continuous data were checked for normality using the Shapiro-Wilk test. Normally distributed data are presented as mean ± SD and were analyzed using unpaired Student’s t-test. Skewed data are expressed as median (Q1, Q3) and analyzed using the Mann-Whitney U test. For repeated measures, a two-way repeated measure analysis of variance (ANOVA) was used to evaluate drug (between-group factor) and time (repeated measures). We analyzed the hemodynamic data for 60-min (shortest intervention time). The Bonferroni test was used to adjust for multiple comparisons. A P value of 0.05 or less was considered significant.
Results
Seventy patients were screened for eligibility, and two patients were excluded because they did not satisfy the study’s inclusion criteria. The 68 included patients were randomized into either NE (n = 34) or PE (n = 34) groups. Six patients (three from each group) were not included in the final analysis. Sixty-two patients were available for the final analysis (31 patients in each group) (Fig. 1).
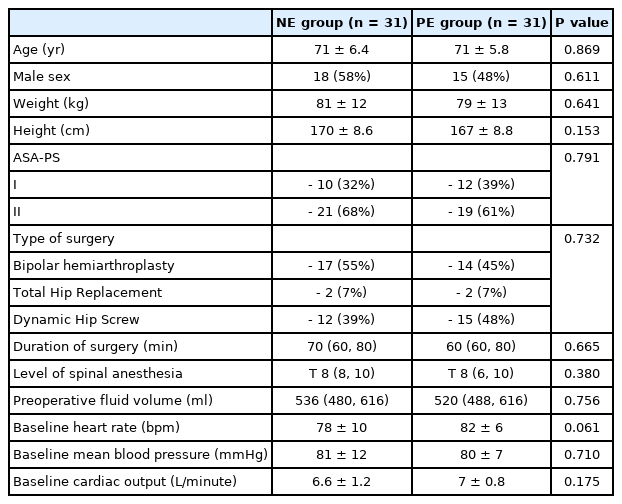
The mean age of the included patients was 71 ± 6 years, and 33 (53%) were men. Demographic data and baseline hemodynamic characteristics were comparable between the study groups (Table 1).
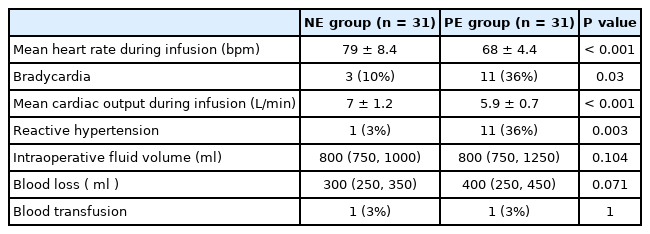
The mean heart rate during vasopressor infusion was higher in the NE group than in the PE group (79 ± 8.4 and 68 ± 4.4 beats/min, respectively, P < 0.001) (Table 2, Fig. 2). The heart rate was generally maintained during NE infusion in relation to the baseline readings. During PE infusion, the heart rate decreased in relation to the baseline reading (Fig. 2). Furthermore, the incidence of bradycardia was lower in the NE group than in the PE group (3/31 [10%] and 11/31 [36%], respectively, P = 0.031) (Table 2). None of the included patients developed tachycardia or hypotension. There was no statistically significant difference in mean blood pressure readings between both groups during drug infusion. The mean blood pressure was higher than the baseline value starting at the eighth and second min after the onset of NE and PE infusion, respectively, until the end of the infusion (Fig. 3). One (3%) patient in the NE group and eleven (36%) patients in the PE group developed reactive hypertension (P = 0.003). (Table 2) The episode of reactive hypertension in the NE group occurred just before the time to terminating the infusion as per the study protocol. The episodes of reactive hypertension in PE usually occurred 2 or 4 min after the onset of PE infusion and lasted for ≤ 2 min and did not require a reduction of the infusion rate.
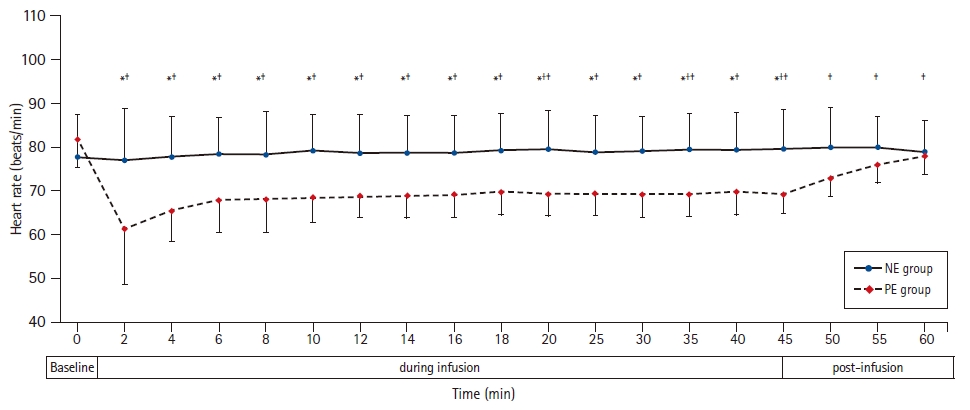
Heart rate at baseline, during and after NE and PE infusions. Markers are mean, while error bars are standard deviations. *Bonferroni-corrected P < 0.05 between the two groups. †Bonferroni-corrected P < 0.05 versus the baseline value in the NE group. ‡Bonferroni-corrected P < 0.05 versus the baseline value in the PE group. NE group: norepinephrine group, PE group: phenylephrine group.
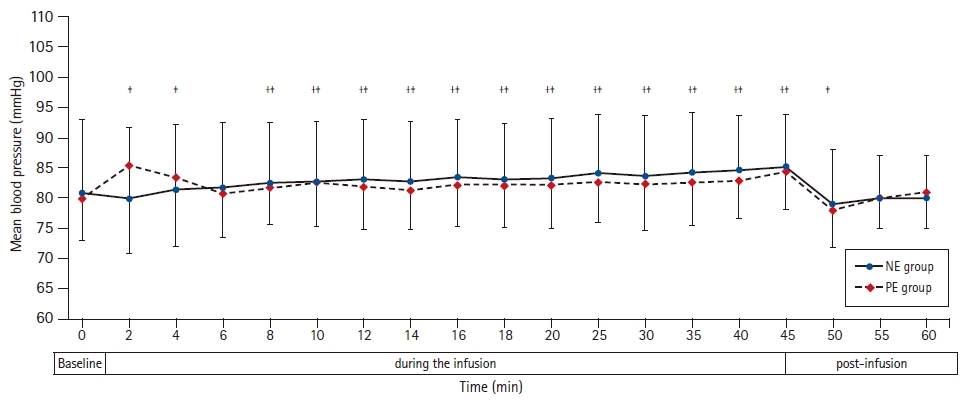
Mean blood pressure at baseline, during and after NE and PE infusions. Markers are mean, while error bars are standard deviations. *Bonferroni-corrected P < 0.05 between the two groups. †Bonferroni-corrected P < 0.05 versus the baseline value in the NE group. ‡Bonferroni-corrected P < 0.05 versus the baseline value in the PE group. NE group: norepinephrine group, PE group: phenylephrine group.
Cardiac output was higher in the NE group than in the PE group. Furthermore, the cardiac output increased in the NE group in relation to the baseline reading, while it decreased in relation to the baseline in the PE group (Table 2, Fig. 4).
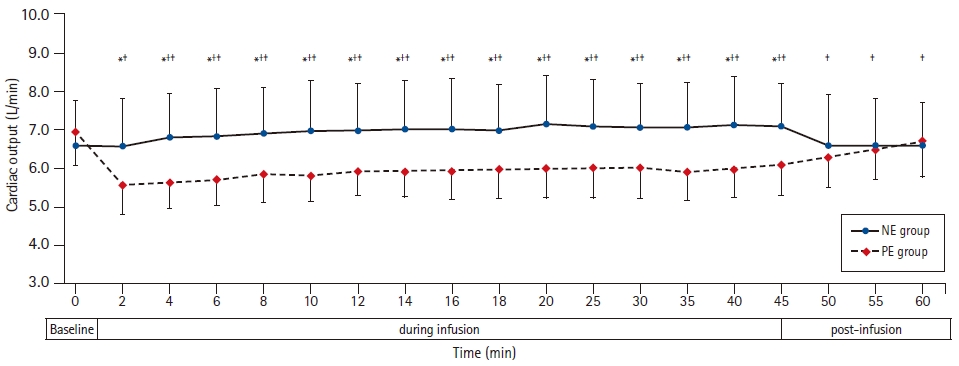
Cardiac output at baseline, during and after NE and PE infusions. Markers are mean, while error bars are standard deviations. *Bonferroni-corrected P < 0.05 between the two groups. †Bonferroni-corrected P < 0.05 versus the baseline value in the NE group. ‡Bonferroni-corrected P < 0.05 versus the baseline value in the PE group. NE group: norepinephrine group, PE group: phenylephrine group.
Immediately after termination of the NE infusion, the heart rate, mean blood pressure, and cardiac output were comparable to the baseline readings. In the PE group, the heart rate and cardiac output continued to be lower in relation to the baseline 15 min after the discontinuation of the infusion (Figs. 2–4).
Other intraoperative outcomes, namely intraoperative fluid volume, blood loss, and the need for blood transfusion, were comparable between the two groups (Table 2).
Discussion
We report that both NE and PE successfully prevented spinal anesthesia-induced hypotension in the elderly. However, NE infusion maintained the heart rate and cardiac output better than PE infusion. The incidence of reactive bradycardia and hypertension was lower, and the mean heart rate was higher in the NE group than in the PE group. These findings are similar to those of previous reports that compared the two drugs in the obstetric population [8,14].
Both NE and PE exert their vascular action through the activation of α-receptors, resulting in vasoconstriction in the arterial and venous sides, increasing systemic vascular resistance and venous return. Owing to its pure α stimulation activity, PE infusion causes a reflex reduction in the heart rate and cardiac output [15] in addition to a direct effect on the left ventricular contractility [16]. In contrast, NE has a modest β-receptor agonist activity, which maintains blood pressure without reducing cardiac output. It has been reported that lower doses of PE could reduce the incidence of reactive hypertension but do not lower the incidence of bradycardia [17,18].
The blood pressure values were comparable between the two study groups. This is probably because we used equipotent doses of the two study drugs. We selected the dose of PE according to Ferré et al. [1], in the same population in which the authors used 100 µg/min to maintain blood pressure. For a fair comparison, we aimed to use an equipotent dose of NE in the other group. Ngan Kee [19] estimated that the relative potency between PE and NE was 1:13; therefore, we hypothesized that an NE dose of 8 µg would be equipotent to a PE dose of 100 µg.
Intraoperative hypotension is an independent risk factor for postoperative 5- and 30-day mortality in patients undergoing hip surgery [2]. Spinal anesthesia, the most common route of anesthesia in hip surgery, is usually associated with hypotension, whose risk is increased in elderly patients. This increased risk is due to age-related cardiovascular changes, such as increased basal sympathetic activity and reduced baroreceptor sensitivity [9]. Furthermore, spinal anesthesia-induced hypotension can occur even in pre-hydrated elderly patients [20,21]. Thus, the use of prophylactic vasopressors for preventing hypotension is increasingly used in vulnerable populations and is already recommended in obstetric practice [22]. PE is the most commonly used drug for prophylaxis and management of postspinal hypotension. However, PE infusion is frequently associated with bradycardia; this was reported in obstetric [8,14] and general surgical patients [16] and was present in 36% of our patients. With advanced age, there is progressive stiffness of the myocardium; hence, the cardiac output is more dependent on active filling from atrial contraction [23]. Therefore, reduction of heart rate in the elderly is associated with reduced cardiac output, which in turn would affect organ oxygen delivery [9]. In the elderly, the main mechanism of spinal anesthesia-induced hypotension is reduced stroke volume [6] and the inability to increase cardiac output [9] rather than a reduction in systemic vascular resistance. In addition to bradycardia, PE has been reported to reduce global left ventricular function [16]. Therefore, it is necessary to use a drug with the least depressing effect on heart rate and, subsequently, the cardiac output.
The heart rate and cardiac output began to increase after discontinuation of the PE infusion but remained lower than the baseline levels until the end of the analysis, while the mean blood pressure returned to baseline level 5 min after terminating the infusion. This delayed return of the heart rate and the cardiac output to the baseline level might be explained by the age-related decrease in baroreceptor sensitivity to changes in blood pressure [24].
The current study introduces, for the first time, NE infusion in the elderly population during spinal anesthesia. By comparing equipotent doses of NE and PE, NE was able to maintain the blood pressure without reducing the heart rate or cardiac output. Since cardiac output is a major determinant of oxygen delivery [25], NE would be able to preserve oxygen delivery in the elderly. By maintaining hemodynamic stability, NE might be beneficial in elderly patients with a high prevalence of impaired myocardial contractility [24,26].
The current study has some limitations. First, it is a single-center study. Second, we did not include patients with cardiac comorbidities. Future research could evaluate the efficacy of both drugs in these patients. Third, we used the available evidence for choosing the equipotent dose of NE to PE, depending on the data obtained from the obstetric population. Future dose-response studies are needed to identify equipotent doses of the two drugs and optimum dosing in the elderly population. Lastly, the cardiac output was assessed noninvasively using electric cardiometry. Electric cardiometry-derived absolute cardiac output values were reported as not interchangeable with the thermodilution method [27]; however, electric cardiometry had acceptable trending ability [28]; thus, it was used to follow-up the changes in the cardiac output in various populations [29,30].
In conclusion, both NE and PE infusions effectively prevented spinal anesthesia-induced hypotension in elderly patients undergoing hip fracture surgery. However, NE provided more hemodynamic stability than PE in the form of maintaining the heart rate, higher cardiac output, less reactive bradycardia, and hypertension. Future dose-response studies are needed to identify the equipotent doses of the two drugs and optimum dosing in the elderly population.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Maha Mostafa Ahmad (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft)
Ahmed Hasanin (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Mahmoud Mostafa (Data curation; Investigation; Methodology; Resources; Writing – original draft; Writing – review & editing)
Mai Y Taha (Data curation; Investigation; Methodology; Resources; Validation; Writing – original draft; Writing – review & editing)
Mohamed Elsayad (Data curation; Investigation; Methodology; Resources; Validation; Visualization; Writing – original draft; Writing – review & editing)
Fatma Alzahraa Haggag (Data curation; Formal analysis; Investigation; Methodology; Resources; Validation; Visualization; Writing – original draft; Writing – review & editing)
Omar Taalab (Conceptualization; Data curation; Investigation; Methodology; Resources; Visualization; Writing – original draft; Writing – review & editing)
Ashraf Rady (Conceptualization; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)
Bassant Abdelhamid (Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing)