 |
 |
| Korean J Anesthesiol > Volume 74(3); 2021 > Article |
|
Abstract
Background
Only a few studies have evaluated the differences between varying concentrations of a fixed dose of local anesthetics. This study was conducted to compare the effects of two different concentrations of a fixed dose of ropivacaine used in ultrasound-guided interscalene brachial plexus block.
Methods
This prospective, randomized, double-blind study included 62 patients who underwent arthroscopic surgery under general anesthesia. The patients were randomly assigned to receive ultrasound-guided interscalene block with 75 mg of ropivacaine at one of two concentrations: 0.75% (10 ml; Group C) or 0.375% (20 ml; Group V). Time to onset of sensory blockade, degree of blockade, pulmonary function changes, analgesic duration of the interscalene block, postoperative opioid requirement within 24 h, postoperative pain scores, satisfaction, and incidence of complications were recorded.
Results
Although the time to onset of sensory blockade was shorter for Group C (P = 0.015), successful blockade was achieved at 30 min after the interscalene block in both groups. The analgesic duration of the interscalene block was not significantly different between the groups. The amount of opioid used within 24 h after surgery was significantly reduced for Group V compared with Group C (P = 0.016). The rest of the parameters did not show any significant differences between the two groups.
Shoulder arthroscopy is a common orthopedic procedure that presents substantial postoperative pain control challenges to the surgeon and the anesthesiologist [1]. Interscalene brachial plexus block (ISB) has been shown effective and is accepted because of its postoperative analgesic and opioid-sparing effects. ISB is therefore central to multimodal postoperative analgesic strategies for these patients [2,3].
Continuous ISB with a catheter insertion may extend the benefits described above. However, routine use of catheters for less invasive shoulder surgeries is impractical and unrealistic and may not be possible in all cases because of the lack of expertise and logistics. In addition, there are risks of catheter dislodgement and infection [3ŌĆō5]. When single-shot ISB is used, the block duration can be an important indicator of clinical efficacy. The duration of ultrasound (US)-guided ISB is reported to be related to the volume and concentration of the local anesthetic (LA) [6]. However, only a few studies have evaluated the differences between varying concentrations of a fixed dose of LA [7,8].
Accordingly, this study was conducted to compare two different concentrations of a fixed dose of ropivacaine when US-guided ISB was performed for pain control after arthroscopic shoulder surgery. This study compared 20 ml of 0.375% ropivacaine, recommended by Fredrickson et al. [9], with 10 ml of 0.75% ropivacaine. It was hypothesized that 75 mg of ropivacaine provided in two different concentration-volume ratios for US-guided ISB would produce different effects for the analgesic duration of ISB.
After Institutional Review Board approval (IRB No. DAUHIRB-19-012, Approved 2019-01-23), this prospective, randomized, double-blind study was registered in cris.nih.go.kr (KCT0003785) on April 15, 2019, prior to patient recruitment. This clinical research was done following the ethical principles for medical research involving human subjects in accordance with the Helsinki Declaration 2013. All patients signed a written consent form before their participation in the study that was conducted from April 18, 2019, to April 23, 2020. Patients aged 18ŌĆō70 years (American Society of Anesthesiologists physical status of ŌģĀ, ŌģĪ, or Ōģó) who were scheduled to undergo arthroscopic shoulder surgery under general anesthesia and had agreed to receive an ISB were enrolled. The exclusion criteria were infection at the ISB site, chronic opioid dependence, morbid obesity (body mass index > 35 kg/m2), pre-existing neurological deficit, chronic obstructive pulmonary disease, coagulopathy, allergy to ropivacaine, uncontrolled diabetes mellitus and/or psychosis, pregnancy or lactation, and refusal to participate. A computer-generated sequence of random numbers and a sealed envelope technique were used to randomize the patients to receive ISB with a fixed dose but different concentrations of ropivacaine as follows: 10 ml of 0.75% ropivacaine (Group C) or 20 ml of 0.375% ropivacaine (Group V).
Intravenous routes were secured for the patients in each ward. Routine monitors (electrocardiogram, non-invasive blood pressure measurement, and pulse oximetry) were attached on arrival of the patient in the operating room. A bedside baseline spirometry was performed for all patients. For bedside spirometry, the patient was placed upright at an angle of 45┬░ on a hospital stretcher, and a Micro handheld spirometer with a disposable mouthpiece (CareFusion, USA) was used. The patient was informed about the procedure, and the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were measured three times; the highest measurement was recorded. All measurements were reassessed 30 min after the ISB to record any changes.
Subsequently, the patient was placed in the lateral decubitus position with the operative shoulder nondependent and the neck extended to facilitate probe positioning. The skin was prepped in a typical sterile fashion, and US-guided ISB was performed using the CX50 device (Philips Ultrasound; USA) with a 12ŌĆō13 MHz, 38-mm linear array transducer (L12-3; Philips). Transverse scanning was performed at the level of the interscalene groove, with the long axis of the probe parallel to the clavicle. The transducer was then moved slightly caudally until the brachial plexus roots were identified. Following confirmation of the transducerŌĆÖs position, 2% lidocaine was injected into the skin to achieve a wheal. Then, a 25-gauge (G), 1.5-inch beveled needle was inserted into the lateral side of the transducer and entered using a lateral-to-medial, in-plane technique. The target position of the needle was the posterior space between the C5 and C6 roots. The position of the needle was confirmed, and 10 ml of 0.75% ropivacaine or 20 ml of 0.375% ropivacaine was slowly injected with intermittent aspiration. When intraneural injection was suspected because of strong resistance during injection or a complaint of paresthesia or pain by the patient, the injection was stopped and the needle was withdrawn and redirected. All ISBs were performed under US guidance alone by a single expert anesthesiologist with experience in the performance of Ōēź 200 blocks.
Neural blockade was evaluated by a clinician who was blinded to the volume and concentration of the injected ropivacaine. Sensory blockade and motor blockade were checked every 3 min for up to 30 min after ISB. Sensory blockade was tested via pinpricks on the C4 (top of the shoulder), C5 (deltoid area), C6 (first fingertip), C7 (middle fingertip), and C8 (little fingertip) dermatomes. The sensory blockade was evaluated using a 3-point verbal rating scale, in which 2, 1, and 0 indicated normal sensation, dull sensation, and absence of sensation, respectively. Motor blockade was evaluated by shoulder abduction (deltoid sign) and forearm flexion, using the modified Bromage scale as follows; 4: full power, 3: reduced power but able to lift the arm against resistance, 2: able to move the muscle group against gravity but unable to lift the arm against resistance, 1: perceptible muscle contraction, but unable to move on purpose, and 0: unable to move the relevant muscle group.
The onset time for a sensory block was defined as the time that elapsed between the end of the ISB procedure and the moment when the pinprick test of the deltoid area yielded a score of 0. Successful blockade was defined as an adequate motor blockade with a score of Ōēż 2 for shoulder abduction and the absence of sensation with pinpricks of the deltoid area. Block failure was considered if shoulder abduction was possible after 30 min or if the pinpricks were felt in the deltoid area; these patients were excluded from the study.
General anesthesia was induced according to the standardized protocol that included intravenous administration of propofol 2ŌĆō2.5 mg/kg and fentanyl 1 ╬╝g/kg with rocuronium 0.6 mg/kg. Anesthesia was maintained with sevoflurane, and the bispectral index was monitored and maintained between 40 and 60. The patient was maintained in a sitting position during surgery. For a hypotensive bradycardic event, defined as intraoperative bradycardia (sitting heart rate [HR] decrease within 5 min by > 30 beats/min compared to the baseline HR, or a decrease to < 50 beats/min at any time) and/or hypotension (sitting systolic blood pressure decrease within 5 min by Ōēź 30 mmHg compared to the baseline pressure, or a decrease to < 90 mmHg at any time), phenylephrine or ephedrine was used by the anesthesiologist, who was blinded to which solution had been injected for ISB. At the end of surgery, remnant neuromuscular blockade was reversed with sugammadex 1ŌĆō4 mg/kg following confirmation of the patientŌĆÖs train-of-four.
Upon extubation, the patient was transferred to the post-anesthesia care unit (PACU). Once the patient was stable and oriented after emergence in the PACU, and a modified Aldrete scale score of > 9 was confirmed, a blinded observer confirmed the pain intensity, measured using the numeric rating scale (NRS; 0 = no pain, 10 = worst possible pain), and the occurrence of Horner syndrome, hoarseness, respiratory distress, postoperative nausea and vomiting (PONV), pneumothorax, dizziness, or paresthesia. After the patient left the PACU, the pain score (at 1, 2, 3, 6, 12, and 24 h) and satisfaction score associated with pain control (at 24 h; 0ŌĆō100) were recorded via a predefined questionnaire. Any complications that occurred within 24 h were also recorded.
After surgery, all patients received ibuprofen (400 mg every 8 h) and acetaminophen (1,000 mg every 8 h) intravenously. Intravenous patient-controlled analgesia (IVPCA) was prepared at a total volume of 100 ml by adding fentanyl 20 ╬╝g/kg and ramosetron 0.6 mg to normal saline. The baseline infusion rate, bolus demand dose, and lock-out time were 1 ml/h, 1 ml, and 10 min, respectively. The IVPCA was initially clamped and first used when the patient complained of pain with an NRS score of Ōēź 4; the time of IVPCA initiation was recorded. The analgesic duration of the ISB was defined as the time between the end of LA injection for the ISB and the postoperative initiation of IVPCA. At 24 h after surgery, the remaining IVPCA volume was assessed, and the fentanyl dosage used until that time was calculated; this marked the end of the study.
The primary outcome was the ISB analgesic duration. A pilot study (n = 10) revealed that the analgesic duration after US-guided ISB with 10 ml of 0.75% ropivacaine in patients who underwent arthroscopic shoulder surgery had a mean ┬▒ SD of 10 ┬▒ 3.8 h. It was hypothesized that a higher volume would result in prolonged analgesic duration of the ISB, and a 20% of time difference was considered clinically important. Considering a type 1 error of 0.05 and a type 2 error of 0.2, 28 patients were considered necessary for each group. Estimating a 10% dropout rate, 31 patients were recruited for each group.
Data were presented as mean ┬▒ SD, median (Q1, Q3), or numbers of patients (%). Quantitative variables were analyzed using the StudentŌĆÖs t test or MannŌĆōWhitney U test, and qualitative variables with the chi-square test or FisherŌĆÖs exact test. The time to the first infusion of IVPCA was analyzed by KaplanŌĆōMeier survival analysis with a comparison between groups using the log-rank test. Survival time was defined as the time from the end of the ISB to the first infusion of IVPCA. All data were analyzed using SPSS┬« software, version 26.0 (SPSS, Inc., USA). Survival curves were plotted using Prism 7.0 for Windows (GraphPad Software, Inc., USA). P < 0.05 was considered statistically significant.ŌĆā
Out of 72 patients assessed for eligibility, seven patients did not meet the inclusion criteria and three patients declined to participate. Sixty-two patients participated in the study; the CONSORT flowchart is shown in Fig. 1. There were no significant between-group differences in the demographic and operative data (Table 1).
The onset of sensory blockade was faster in Group C (8.0 ┬▒ 2.9 min) compared with Group V (10.0 ┬▒ 3.1 min) (P = 0.015). Successful blockade was achieved at 30 min after ISB in all patients. There were no differences between the groups in sensory blockade or motor blockade 30 min after ISB (Table 2). Pulmonary function change assessed by FEV1 and FVC did not differ between the groups (Table 3).
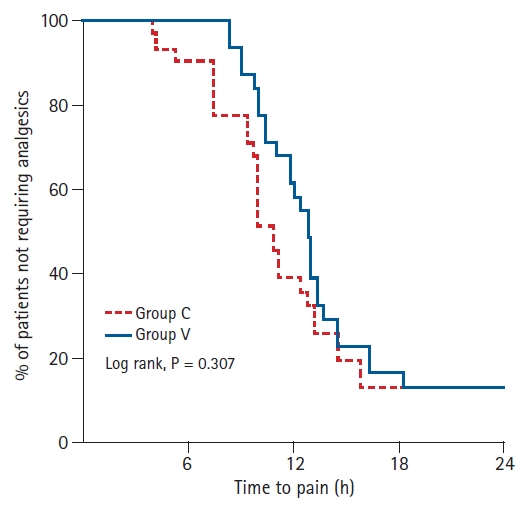
There was no significant difference between the groups for the number of patients who did not require IVPCA analgesia within 24 h after surgery (Fig. 2). The ISB analgesic duration was not significantly different between the groups (Group C: 12.1 ┬▒ 5.4 h vs. Group V: 13.7 ┬▒ 4.7 h; P = 0.214). The total amount of fentanyl used within 24 h after surgery was significantly reduced for Group V (248.3 ┬▒ 112.2 ╬╝g) compared with Group C (331.3 ┬▒ 149.9 ╬╝g) (P = 0.016). Group V exhibited a reduction in the cumulative dose of the baseline infusion and the bolus demand for fentanyl within 24 h (Table 3).
To our knowledge, this is the first study to compare the use of two different concentrations of a fixed dose of ropivacaine (75 mg) for US-guided ISB. The results showed no difference in pulmonary function change between the two groups, while the onset of sensory blockade was faster in Group C. There was no difference in analgesic duration of the ISB between the two groups. However, postoperative opioid requirement within 24 h was reduced in Group V.
A previous study comparing 1.0 ml/kg of 0.225% and 1.5 ml/kg of 0.15% ropivacaine for caudal analgesia for pediatric orchiopexy reported that the latter formulation produced a higher level of block and provided better quality and longer duration of analgesia [10]. However, 20 ml of 0.375% ropivacaine did not result in longer analgesic duration compared with 10 ml of 0.75% ropivacaine in our study. The most important possible factor for this difference is the anatomical characteristics surrounding the target nerve. Caudal analgesia regresses from the site of lowest anesthetic concentration distal to the injection site and caudally toward the highest concentration that can be explained by diffusion and uptake into the surrounding vasculature [10]. Compared with this model, the anatomical structures that surround the ISB target point have several characteristics: more openness, less firmness to prevent the spread of LA from the target nerves, more variable surrounding vessels that contribute to regression of LA, and a higher nonneural-neural tissue ratio [11]. These characteristics may produce a more complicated relationship between the volume of LA and analgesic duration of the ISB compared with caudal analgesia. Another dose-finding study reported a reduced ED(dose)95 when a constant volume was set, and the concentration was lowered, than when a constant concentration was set and the volume was lowered [9]. Just as individual patients may respond differently to the same dose of the same drug delivered in exactly the same location in relation to the target nerves, the accuracy with which the LA drug can be deposited might differ between patients. It was expected that volume rather than concentration would have an advantage to prolong the analgesic duration that was not found in our study. The concept of the mean effective volume (MEV) and minimum effective anesthetic concentration (MEAC) may be more important than the ideal combination of volume and concentration in terms of analgesic efficacy of peripheral nerve block. If a well-experienced clinician uses the doses of the MEV and MEAC, then the accuracy of the LA deposit in relation to volume would no longer be significant.
Zhai et al. [7] evaluated US-guided ISB performed with different volumes of a 50 mg dose of ropivacaine and found that a higher concentration of ropivacaine was associated with faster onset of sensory blockade, as observed in this study. Although our study did not confirm the length of stay in the operating room and PACU, the results suggest that the higher concentration may be preferable to facilitate the surgery and reduce the overall processing time. However, this trend could not be verified, because our study compared only two groups that received one fixed dose. Further studies are necessary to verify the maintenance of this trend.
Although the difference in analgesic duration of the ISB between groups was not statistically significant, it could have influenced the postoperative opioid requirement within 24 h, in which the pain relief regimen primarily comprised single-shot ISB and IVPCA, reflected by the lower baseline-infused fentanyl dose in Group V. Bolus demand was greater in Group C compared with Group V. Nerve injury or neurotoxicity is one of the possible factors for rebound pain after peripheral nerve block; therefore, reducing the concentration of LA would be beneficial [12,13]. Reducing the concentration of ropivacaine rather than lowering volume could be associated with less rebound pain. To summarize, our results could be clinically significant for a postoperative pain control regimen centered on single-shot ISB and IVPCA within 24 h, during which the patients experienced the most severe pain.
ISB is associated with decreased pulmonary function due to ipsilateral phrenic nerve palsy [14,15]. Compared with previously published low-dose studies, our study used a relatively high dose; therefore, we expected to find a nearly 100% incidence of decreased pulmonary function in our study [14ŌĆō17]. Only two patients in Group C and one in Group V showed no reduction in FEV1 and FVC. These patients might not have had phrenic nerve palsy, although this could not be confirmed, because direct US evaluation was not performed. In the pilot study, evaluation of diaphragmatic movements was attempted by the use of US. Because of the difficulties encountered with the use of the spleen as a window to identify the left diaphragm, this factor was excluded from analysis [18]. Even so, our study showed a similar probability of reduced pulmonary function as previous studies.
A previous study showed that a high volume/low concentration combination of LA for ISB could avoid major complications [8]; however, the evidence seemed weak because the study used a conventional LA dose and a multi-injection technique with nerve stimulation. Thus, the LA volume variation was not an isolated independent factor. There was no significant between-group difference for the incidence of complications in our study. Previous studies showed that doses as low as 5 ml could lead to a reduction of ISB-associated complications [16,17]. Because ISB complications are related to the surrounding anatomy (e.g., HornerŌĆÖs syndrome ŌĆō stellate ganglion, hemidiaphragmatic paralysis ŌĆō phrenic nerve, hoarseness ŌĆō recurrent laryngeal nerve), the incidence of block-related complications can be expected to decrease only when the dose is low enough to prevent hemidiaphragmatic paralysis.
This study has several limitations. First, because the anesthesiologist who performed the procedure used a different volume of ropivacaine, correction of maldistribution of the injected LA might have been more ideal for Group V. However, considering that the US-guided ISB was performed by a single expert anesthesiologist, the need for repositioning of the needle was reduced. Second, sensory and motor blockades after surgery were not assessed that could affect the patientŌĆÖs satisfaction score. However, the surgeon at the hospital wanted the shoulders and arms of the patients kept immobile in the early postoperative stage. Consequently, accurate evaluation was almost impossible because of the dressing or abduction brace. Third, the incidence of PONV was likely to be influenced by both the ISB and IVPCA, and not by ISB alone. However, the frequency was the same in both groups; therefore, it was unlikely to have a significant influence on the overall result.
In conclusion, compared with 10 ml of 0.75% ropivacaine, 20 ml of 0.375% ropivacaine did not prolong analgesic duration of the ISB. Nevertheless, it might be effective to reduce the postoperative opioid requirement within 24 h when combined with IVPCA for analgesia after arthroscopic shoulder surgery.
NOTES
Author Contributions
Seung Cheol Lee (Conceptualization; Supervision)
Joon Ho Jeong (Investigation; Writing ŌĆō original draft)
Seong Yeop Jeong (Data curation)
Sung Wan Kim (Data curation)
Chan Jong Chung (Supervision; Writing ŌĆō review & editing)
So Ron Choi (Formal analysis)
Jeong Ho Kim (Investigation)
Sang Yoong Park (Investigation; Writing ŌĆō review & editing)
Fig.┬Ā1.
CONSORT diagram of patient flow through the study. ISB: interscalene brachial plexus block.

Fig.┬Ā2.
Proportion of patients not requiring analgesics in relation to time after block in the groups receiving 10 ml of 0.75% ropivacaine (Group C) and 20 ml of 0.375% ropivacaine (Group V). There was no significant difference between the groups (P = 0.307, log-rank test).
Table┬Ā1.
PatientŌĆÖs Characteristics and Operative Data
Table┬Ā2.
Sensory and Motor Block Assessment after 30 min after Interscalene Block
| Variable | Group C (n = 31) | Group V (n = 31) | P value |
|---|---|---|---|
| Performance time (min) | 9.0 ┬▒ 1.8 | 9.0 ┬▒ 1.9 | > 0.999 |
| Time to onset of a sensory block (min) | 8.0 ┬▒ 2.9 | 10.0 ┬▒ 3.1 | 0.015 |
| Sensory block (0/1/2)* | |||
| ŌĆāC4 | 12/15/4 | 11/16/4 | > 0.999 |
| ŌĆāC5 | 31/0/0 | 31/0/0 | > 0.999 |
| ŌĆāC6 | 22/9/0 | 21/10/0 | 0.783 |
| ŌĆāC7 | 5/21/5 | 10/16/5 | 0.310 |
| ŌĆāC8 | 0/19/12 | 0/16/15 | 0.442 |
| Motor block (0/1/2/3/4)ŌĆĀ | |||
| ŌĆāShoulder abduction | 19/12/0/0/0 | 20/11/0/0/0 | 0.793 |
| ŌĆāForearm flexion | 17/14/0/0/0 | 12/17/2/0/0 | 0.217 |
Table┬Ā3.
Secondary Outcomes according to Block during 24 h after Surgery
Table┬Ā4.
Postoperative Pain Scores by Time Period
References
1. Hughes MS, Matava MJ, Wright RW, Brophy RH, Smith MV. Interscalene brachial plexus block for arthroscopic shoulder surgery: a systematic review. J Bone Joint Surg Am 2013; 95: 1318-24.

2. Singh A, Kelly C, OŌĆÖBrien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am 2012; 94: 2040-6.


3. Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A systematic review and meta-analysis. Anesth Analg 2015; 120: 1114-29.


4. Fredrickson MJ, Krishnan S, Chen CY. Postoperative analgesia for shoulder surgery: a critical appraisal and review of current techniques. Anaesthesia 2010; 65: 608-24.


5. Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL. Peripheral nerve blocks in the management of postoperative pain: challenges and opportunities. J Clin Anesth 2016; 35: 524-9.


6. Fredrickson MJ, Abeysekera A, White R. Randomized study of the effect of local anesthetic volume and concentration on the duration of peripheral nerve blockade. Reg Anesth Pain Med 2012; 37: 495-501.


7. Zhai W, Wang X, Rong Y, Li M, Wang H. Effects of a fixed low-dose ropivacaine with different volume and concentrations on interscalene brachial plexus block: a randomized controlled trial. BMC Anesthesiol 2016; 16: 80.



8. Pippa P, Cuomo P, Panchetti A, Scarchini M, Poggi G, DŌĆÖArienzo M. High volume and low concentration of anaesthetic solution in the perivascular interscalene sheath determines quality of block and incidence of complications. Eur J Anaesthesiol 2006; 23: 855-60.


9. Fredrickson MJ, Smith KR, Wong AC. Importance of volume and concentration for ropivacaine interscalene block in preventing recovery room pain and minimizing motor block after shoulder surgery. Anesthesiology 2010; 112: 1374-81.


10. Hong JY, Han SW, Kim WO, Cho JS, Kil HK. A comparison of high volume/low concentration and low volume/high concentration ropivacaine in caudal analgesia for pediatric orchiopexy. Anesth Analg 2009; 109: 1073-8.


11. Vadhanan P, Tripaty DK, Adinarayanan S. Physiological and pharmacologic aspects of peripheral nerve blocks. J Anaesthesiol Clin Pharmacol 2015; 31: 384-93.



12. Dada O, Gonzalez Zacarias A, Ongaigui C, Echeverria-Villalobos M, Kushelev M, Bergese SD, et al. Does rebound pain after peripheral nerve block for orthopedic surgery impact postoperative analgesia and opioid consumption? A narrative review. Int J Environ Res Public Health 2019; 16: 3257.



13. Farber SJ, Saheb-Al-Zamani M, Zieske L, Laurido-Soto O, Bery A, Hunter D, et al. Peripheral nerve injury after local anesthetic injection. Anesth Analg 2013; 117: 731-9.


14. Sinha SK, Abrams JH, Barnett JT, Muller JG, Lahiri B, Bernstein BA, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med 2011; 36: 17-20.


15. Lim YC, Koo ZK, Ho VW, Chang SS, Manohara S, Tong QJ. Randomized, controlled trial comparing respiratory and analgesic effects of interscalene, anterior suprascapular, and posterior suprascapular nerve blocks for arthroscopic shoulder surgery. Korean J Anesthesiol 2020; 73: 408-16.

16. Riazi S, Carmichael N, Awad I, Holtby RM, McCartney CJ. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth 2008; 101: 549-56.











