 |
 |
| Korean J Anesthesiol > Volume 74(1); 2021 > Article |
|
Abstract
Background
Cytoreductive surgery was developed as a treatment for peritoneal carcinomatosis. However, this surgery is associated with important complications. The present study aimed to assess the relationship between lactacidemia and the rate of associated complications during the immediate postoperative period in the intensive care unit (ICU) in patients undergoing cytoreductive surgery.
Methods
This was a retrospective observational study. A total of 57 patients underwent cytoreductive surgery. All patients were admitted to the ICU immediately after the surgery. Data on lactic acid levels at the time of admission and discharge from the ICU were collected. Postsurgical complications that occurred during the ICU stay were recorded according to failure-to-rescue analysis and their severity stratified according to the Clavien-Dindo classification.
Results
The lactic acid levels at admission to the ICU were significantly higher in patients who developed complications, with an almost tripled unadjusted relative risk (2.9, 95% CI: 1.6, 5.3), than in those who did not develop complications for the lactacidemia threshold established in the cumulative sum curve graphs. After adjustment for confounding effects, the relative risk became even higher (3.1, 95% CI: 1.8, 3.6). Lactic acid levels were still significantly higher in this group at the time of discharge from the ICU.
Conclusions
Serum lactate level is a risk factor for postoperative complications in patients undergoing cytoreductive surgery for peritoneal carcinomatosis. This study suggests that the risk of developing severe complications almost triples with a lactic acid level of 2.5 mmol/L or higher at the time of admission in the ICU.
Cytoreductive surgery (CRS), associated or not with hyperthermic intraperitoneal chemotherapy (HIPEC), was developed in the last decade as a therapeutic option for selected patients with peritoneal carcinomatosis. Historically, peritoneal carcinomatosis has been considered an advanced and incurable disease [1,2]. The objective of CRS is to attempt to eradicate the microscopic disease and reduce peritoneal recurrence, following the precepts initially described by Sugarbaker [3]. This strategy has been reported to significantly improve the survival of patients with this condition [4]. However, in performing complex procedures, the associated morbidity and mortality must be taken into account [5]. CRS associated or not with HIPEC requires close monitoring throughout the entire perioperative period for optimal management.
Elevated levels of lactic acid (lactacidemia) has been shown to be correlated with tissue hypoxia and to predict increased perioperative morbidity and mortality [6,7]. According to Spiliotis et al. the average of 3 and 4 postoperative day lactate level is an independent predictor of morbidity and mortality in patients undergoing CRS and HIPEC. Our study shows a definite threshold in lactacidemia levels for an increased risk of complications.
Hence, the objective of this study was to evaluate the relationship between lactacidemia and the rate of associated complications during the immediate postoperative period in the intensive care unit (ICU) at the University General Hospital of Castellón between 2014 and 2016 in patients undergoing CRS.
All cases during a 2-year period (2014–2016) were included, which corresponded to the initial series of patients diagnosed with peritoneal carcinomatosis who underwent CRS in the Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery of the University General Hospital of Castellón. All procedures were performed by the same surgical team. Information on the patients’ clinical and pathological characteristics, surgical procedures, and residual disease at surgery were prospectively collected and retrospectively analyzed for the purpose of this study.
All patients were admitted during the immediate postoperative period in the ICU of our hospital. The study was approved by the Institutional Review Board of the University General Hospital of Castellón on June 25, 2019 (approval number: HGCLAC01). The study is registered in the Clinical Trials Registry (Registration number: NCT04307654). The clinical research was done following the ethical principles for medical research involving human subjects in accordance with the Helsinki Declaration 2013.
Data on lactic acid levels, measured using arterial blood samples, were collected at the time of admission and discharge from the ICU. Postsurgical complications that developed during the stay in the ICU were recorded according to failure-to-rescue analysis [8] (Table 1) and their severity stratified according to the Clavien-Dindo classification [9]. When several complications occurred in the same patient, the complication with the highest degree was considered.
Quantitative variables are summarized as median and interquartile range or mean ± SD. Categorical variables are presented using relative frequencies and percentages. For inferential analysis, the Mann-Whitney U test and Fisher’s exact test were used, as appropriate. Cumulative sum curves (CUSUMs) were used to obtain the most discriminating cutoff point of lactic acid level in relation to complications. These are the cumulative differences between an expected result, the general prevalence of complications, and the result observed in each case. Thus, these curves reveal the changes in the trend of the result of interest through different values of the prediction variable [10].
An adjustment for confounding effects was performed with logistic regression using the multivariate propensity score method. Possible confounding factors were considered, including Charlson age score, presence of ascites, current tumor (primary or relapsed), carcinomatosis index [11], number of visceral resections, blood loss, and norepinephrine dosage.
Statistical analyses were performed with the statistical program STATA version 15.1 (Stata Corp., USA).
During 2014 and 2016, a total of 57 patients underwent CRS. The demographics of the patients and the disease are shown in Table 2. Ovarian tumors (stages IIIC and IV International Federation of Gynecology and Obstetrics) were the most prevalent. Most of the patients scheduled for this surgery did not have significant comorbidity, and a carcinomatosis index of > 20 was observed in only 1 of every 5 cases. No major demographical differences were detected between patients who developed complications in the ICU and those who did not (Table 2).
Table 3 summarizes the surgical treatment factors that could influence the incidence of postoperative complications, as well as the oncological results of surgery and postoperative morbidity and mortality. No significant differences were detected between the groups without and with complications with respect to the type of surgery. However, in patients without complications in the ICU, the degree of complications that appeared in the surgical ward was significantly lower than in those who developed complications in the ICU. All 4 (7%) deaths in the series occurred during the stay in the ICU, which represents 14% of patients who developed complications in the ICU.
The lactic acid levels at admission to the ICU were significantly higher in those who presented complications, with an unadjusted odds ratio of 10.1 (95% CI: 2.5, 44) and an almost tripled unadjusted relative risk (2.9, 95% CI: 1.6, 5.3), than in those without complications for the threshold of lactacidemia established in the CUSUM graphs. After adjustment for confounding effects using multivariate propensity scores, the adjusted odds ratio became 16 (95% CI: 2.4, 103) and the adjusted relative risk was 3.1 (95% CI: 1.8, 3.6). Fig. 1 shows that, at the time of discharge from the ICU, the lactic acid levels were still significantly higher in the group of patients who presented complications. Fig. 2 shows the levels of lactic acid in the group of patients who did not develop complications.
It was observed in the CUSUM graphs that a lactic acid level of 2.5 mmol/L marks the threshold for an increased risk of complications. Fig. 3 shows the relationship between lactacidemia at admission in ICU and the development of complications. Fig. 4 shows the relationship between lactacidemia at admission in the ICU and death. The inflexion in the curves from that point means that the observed complications and deaths exceeded the expected values; the expected-observed difference negatively accumulated with statistical significance (P < 0.001 for complications; P = 0.02 for mortality) as lactic acid levels increased. The predominant grade of complications for levels up to 2.5 mmol/L was grade II (complications that do not require intervention), whereas it was grade III for higher levels, with grade IV complications and the 4 deaths of the series also appearing in this cohort. These data are shown in Table 4.
The analysis of our study data indicated that the level of lactate is an important predictor of postsurgical complications.
Lactate is produced by most of the tissues in the human body, with muscle tissue being the main production site [12]. Under normal conditions, lactate is quickly cleared by the liver and kidneys. During glycolysis, under aerobic conditions, pyruvate is oxidized via pyruvate dehydrogenase to acetyl-CoA that enters the Krebs cycle, obviating lactate production. In anaerobic conditions, pyruvate dehydrogenase is inhibited and pyruvate becomes lactate. Under these circumstances, lactate is the final product of glycolysis and becomes a substrate for gluconeogenesis in the Cori cycle. Its production is increased under hypoperfusion or stress conditions, causing an increase in glycolysis [12–14]. Although lactate levels may be elevated in several conditions, it has been effectively used as a measure of tissue hypoxia [1,6,15]. However, this is not uniformly accepted, as the level may increase under other conditions [13,14,16,17].
The findings of this study suggested that the risk of developing severe complications almost triples with a lactic acid level of 2.5 mmol/L or higher at the time of admission in the ICU, when the anesthetic phase has just ended.
Several studies have evaluated the clinical use of lactate level in postoperative patients [7,18,19]. The effectiveness of lactate level as a marker of morbidity and mortality in the postoperative period of patients undergoing CRS has also been demonstrated [6]. Our study confirms this relationship between lactic acid level and the development of complications.
Our findings are compatible with those of other studies [19–21]. In a prospective study that included 88 patients undergoing major abdominal surgery, it was observed that patients with elevated lactate levels had a significantly higher complication rate than patients without incidents in the postoperative course and whose lactate levels were in the normal range [19]. Another study found an association between blood lactate levels and organ failure and death [20]. Likewise, other studies indicated that surgical patients who develop complications have a greater deficit of tissue oxygenation during the intervention [21].
Previous studies have employed lactate level as a measure of tissue hypoperfusion, using its clearance as a resuscitation guide [13,14]. Those studies indicated that not only the increase in lactate levels but also the time until normalization of the levels were associated with postoperative morbidity and mortality. The relationship between lactic acid elevation and postsurgical morbidity in CRS has also been shown by Spiliotis et al. [6] in a study on patients undergoing CRS and HIPEC. The authors did not find an association between intraoperative lactic aid levels and postoperative morbidity and mortality. However, they highlighted the clinical relevance of lactate measurement on postoperative days 3 and 4, in that an increase of 1 mmol/L in the average lactate value on days 3 and 4 increases the risk of a minor complication by 1.9, the risk of a major complication by 10.9, and the risk of mortality by 32.1%. In our study, the risk of developing complications almost tripled when lactate levels were > 2.5 mmol/L.
One of the strengths of the present study lies on the homogeneity of the cohort of patients with abdominal carcinomatosis who were treated by the same specialized multidisciplinary team. Further, it was possible to find a clear threshold of lactic acid level that discriminates the outcomes in terms of morbidity and mortality.
With respect to the limitations of this study, one was its observational design. The data were from a small series and from a single center during the immediate postoperative period in the ICU, thus making generalization impossible, although they supported the findings of similar studies. Our data did not show statistical differences in lactate levels with respect to surgery duration, disease stage, or patients’ comorbidities, probably because of the small sample size. Further, it has been hypothesized that several incidents during anesthesia [22–24] result in lactate elevation. This analysis would be strengthened by incorporating earlier time points (including intraoperative time points) of lactic acid measurement. In the future, analyzing these details of the anesthetic procedure constitutes an interesting research field. Similarly, data that relate the delay in the clearance of lactate to a greater number of complications were not available; measuring the rate at which lactic acid levels do (or do not) improve is another information that needs investigation.
Serum lactate level is a predictive factor for postoperative complications in patients undergoing CRS for peritoneal carcinomatosis. More studies with a larger number of patients and with more preoperative (liver function, renal function, drugs that interact with liver function), intraoperative (complexity of surgery, peritoneal carcinomatosis index, tissue oxygen supply, central oxygen saturation, anesthetic incidents), and postoperative (lactate clearance index) data should be performed to fully elucidate this phenomenon.
Acknowledgments
The research team would like to thank all the members of MUAPOS group (residents, nursing staff, doctors) for the work developed.
NOTES
Author Contributions
Marta Soriano Hervás (Writing – original draft)
Rosa Játiva-Porcar (Investigation)
Daniel Robles-Hernández (Investigation)
Anna Serra Rubert (Investigation)
Blanca Segarra (Investigation)
Cristina Oliva (Investigation)
Javier Escrig (Data curation; Formal analysis; Investigation; Methodology; Writing – review & editing)
José Antonio Llueca (Data curation; Formal analysis; Investigation; Writing – review & editing)
Fig. 1.
Level of lactic acid in the group of patients who developed complications in the intensive care unit (ICU).

Fig. 2.
Level of lactic acid in the group of patients who did not develop complications in the intensive care unit (ICU).

Fig. 3.
Cumulative sum curve (CUSUM) for complications. The arrow points to the most discriminating cutoff point of lactic acid level with respect to the presence of complications from each value of lactic acid level. ICU: intensive care unit.
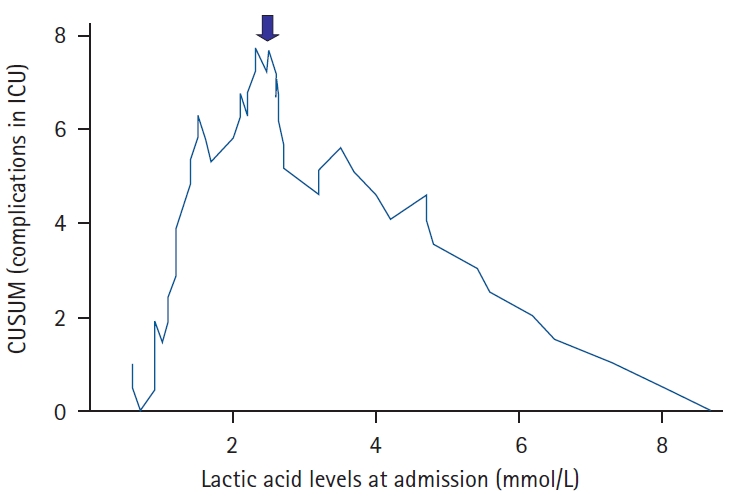
Fig. 4.
Cumulative sum curve (CUSUM) for death. The arrow points to the most discriminating cutoff point of lactic acid level with respect to death from each value of lactic acid level. ICU: intensive care unit.

Table 1.
Complications Used in Traditional Failure-to-rescue Analysis
From Silber et al. [8].
Table 2.
Patient Demographics
| Variable | Total series (n = 57) |
Complications in ICU |
P value | |
|---|---|---|---|---|
| No (n = 29) | Yes (n = 28) | |||
| Age (yr) | 61 (52, 67) | 64 (58, 66) | 60 (50, 67.5) | 0.297* |
| Current tumor | 0.610† | |||
| Primary | 41 (72) | 20 (69) | 21 (75) | |
| Relapsed | 16 (28) | 9 (31) | 7 (25) | |
| Carcinomatosis type | 0.849† | |||
| Colorectal | 2 (4) | 1 (3) | 1 (4) | |
| Ovarian | 47 (82) | 24 (83) | 23 (82) | |
| Endometrial | 2 (4) | 1 (3) | 1 (4) | |
| Pseudomyxoma | 1 (2) | 1 (3) | 0 (0) | |
| Primary | 4 (7) | 2 (7) | 2 (7) | |
| Gastric | 1 (2) | 0 (0) | 1 (4) | |
| Neoadjuvant treatment | 21 (37) | 8 (28) | 13 (46) | 0.141† |
| Carcinomatosis index | 0.469† | |||
| 1–10 | 26 (46) | 11 (38) | 15 (54) | |
| 11–20 | 20 (35) | 12 (41) | 8 (29) | |
| +20 | 11 (19) | 6 (21) | 5 (18) | |
| CA125 | 174 (51, 800) | 174 (58, 1280) | 177.5 (50, 500) | 0.672* |
Table 3.
Procedure Characteristics
| Variable | Total series (n = 57) |
Complications in ICU |
P value | |
|---|---|---|---|---|
| No (n = 29) | Yes (n = 28) | |||
| Digestive anastomosis | 39 (68) | 20 (69) | 19 (68) | 0.928† |
| Lymphadenectomy | 47 (82) | 25 (86) | 22 (79) | 0.451† |
| HIPEC | 19 (33) | 10 (34) | 9 (32) | 0.849† |
| Duration (min) | 480 (400, 600) | 540 (410, 600) | 458 (375, 585) | 0.230* |
| Visceral resections | 48 (84) | 25 (86) | 23 (82) | 0.673† |
| Number of resections | 4 (3, 6) | 4 (3, 6) | 4 (2.5, 5) | 0.519* |
| Blood loss (ml) | 0.451† | |||
| 0–1000 | 7 (12) | 5 (17) | 2 (7) | |
| 1000–2000 | 20 (35) | 8 (28) | 12 (43) | |
| 2000–3000 | 25 (44) | 14 (48) | 11 (39) | |
| +3000 | 5 (9) | 2 (7) | 3 (11) | |
| Cytoreduction | 0.172† | |||
| CC-0 | 49 (86) | 25 (86) | 24 (86) | |
| CC-1 | 4 (7) | 3 (10) | 1 (4) | |
| CC-2 | 3 (5) | 0 (0) | 3 (11) | |
| CC-3 | 1 (2) | 1 (3) | 0 (0) | |
| Lactic acid level at ICU admission (mmol/L) | 2.3 (1.2, 3.2) | 1.4 (1.1, 2.3) | 2.7 (2.25, 5.1) | < 0.001* |
| Lactic acid level at ICU discharge (mmol/L) | 0.8 (0.6, 0.8) | 0.6 (0.5, 0.8) | 0.8 (0.7, 0.9) | 0.020* |
| Noradrenaline (μg/kg/min) | 0.3 (0.15, 0.6) | 0.2 (0.1, 0.4) | 0.3 (0.28, 0.8) | 0.021* |
| Clavien-Dindo | 0.002† | |||
| No complication | 11 (19) | 10 (35) | 1 (4) | |
| Grade I | 2 (4) | 0 (0) | 2 (7) | |
| Grade II | 19 (33) | 11 (38) | 8 (29) | |
| Grade III | 19 (33) | 8 (27) | 11 (39) | |
| Grade IV | 2 (4) | 0 (0) | 2 (7) | |
| Grade V (death) | 4 (7) | 0 (0) | 4 (14) | |
| Complications in ward | 31 (54) | 18 (62) | 13 (46) | 0.240† |
| Accumulated complications by patient (ICU + ward) | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.599* |
| Death in ICU | 4 (7) | 4 (14) | 0.035 | |
| Death in ward | 0 (0) | 0 (0) | 0.99 | |
Table 4.
Morbidity and Mortality in the ICU according to Lactic Acid Level
Table 5.
Patients’ Features and Operative Variables according to Lactic Acid Level at Admission to the Intensive Care Unit
Values are presented as mean ± SD or frequency (%). CPI: carcinomatosis peritoneal index [11].
References
1. Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg 1996; 171: 221-6.


2. Spiliotis J, Halkia E, de Bree E. Treatment of peritoneal surface malignancies with hyperthermic intraperitoneal chemotherapy-current perspectives. Curr Oncol 2016; 23: e266-75.




3. Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 1999; 43 Suppl: S15-25.


4. López-López V, Cascales-Campos PA, Schneider MA, Gil J, Gil E, Gomez-Hidalgo NR, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in elderly patients. A systematic literature review. Surg Oncol 2016; 25: 378-84.


5. Llueca A, Serra A, Maiocchi K, Delgado K, Jativa R, Gomez L, et al. Predictive model for major complications after extensive abdominal surgery in primary advanced ovarian cancer. Int J Womens Health 2019; 11: 161-7.



6. Spiliotis J, Halkia E, Zouridis A, Vassiliadou D, Zakka M, Kalantzi N, et al. Serum lactate as predictor of morbidity, mortality and long term survival in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Case Stud Surg 2015; 1: 41-6.

7. Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg 2003; 185: 485-91.


8. Silber JH, Romano PS, Rosen AK, Wang Y, Even-Shoshan O, Volpp KG. Failure-to-rescue: comparing definitions to measure quality of care. Med Care 2007; 45: 918-25.


9. Mitropoulos D, Artibani W, Graefen M, Remzi M, Rouprêt M, Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Actas Urol Esp 2012; 37: 1-11.


10. Royston P. The use of cusums and other techniques in modelling continuous covariates in logistic regression. Stat Med 1992; 11: 1115-29.


11. Llueca A, Escrig J. Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur J Surg Oncol 2018; 44: 163-9.


12. Rosenstein PG, Tennent-Brown BS, Hughes D. Clinical use of plasma lactate concentration. Part 1: Physiology, pathophysiology, and measurement. J Vet Emerg Crit Care (San Antonio) 2018; 28: 85-105.

13. McNelis J, Marini CP, Jurkiewicz A, Szomstein S, Simms HH, Ritter G, et al. Prolonged lactate clearance is associated with increased mortality in the surgical intensive care unit. Am J Surg 2001; 182: 481-5.


14. Weil MH, Afifi AA. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 1970; 41: 989-1001.


16. Gore DC, Jahoor F, Hibbert JM, Demaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen availability. Ann Surg 1996; 224: 97-102.



17. Steffes CP, Dahn MS, Patricia MP. Oxygen transport-dependent splanchnic metabolism in the sepsis syndrome. Arch Surg 1994; 129: 46-52.


18. Bini R, Ferrari G, Aprà F, Viora T, Leli R, Cotogni P. Peritoneal lactate as a potential biomarker for predicting the need for reintervention after abdominal surgery. J Trauma Acute Care Surg 2014; 77: 376-80.


19. Li S, Peng K, Liu F, Yu Y, Xu T, Zhang Y. Changes in blood lactate levels after major elective abdominal surgery and the association with outcomes: a prospective observational study. J Surg Res 2013; 184: 1059-69.


20. Roumen RMH, Redl H, Schlag G, Sandtner W, Koller WA, Goris RJ. Scoring systems and blood lactate concentrations in relation to the development of adult respiratory distress syndrome and multiple organ failure in severely traumatized patients. J Trauma 1993; 35: 349-55.


21. Shoemaker WC, Appel PL, Kram HB. Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med 1988; 16: 1117-20.


22. Lees N, Hamilton M, Rhodes A. Clinical review: goal-directed therapy in high risk surgical patients. Crit Care 2009; 13: 231.












