 |
 |
| Korean J Anesthesiol > Volume 72(5); 2019 > Article |
|
Abstract
Background
Sugammadex is a reversal agent for non-depolarizing neuromuscular blockers and widely used worldwide on account of its rapid and effective reversal from neuromuscular blockade, despite its advantages, multiple cases of sugammadex-induced anaphylactic shock have been reported.
Case
A 42-year-old man developed anaphylactic shock in the postanesthesia care unit. Initially, sugammadex was suspected as the causative agent, but an intradermal skin test revealed negative results. A further skin test was performed with sugammadex-rocuronium complex that yielded positive results.
Sugammadex is the first non-competitive and selective reversal agent for an aminosteroid non-depolarizing neuromuscular blocker. Despite its rapid and effective reversal from neuromuscular blockade, sugammadex was prohibited by the US Food and Drug Administration due to concerns of life-threatening hypersensitivity reactions until late 2015. Multiple cases of sugammadex-induced anaphylactic shock have been reported [1]. Furthermore, although the sugammadex-rocuronium complex has been regarded as biologically inactive, it may cause clinical anaphylaxis. Herein, we report a case of anaphylactic shock after sugammadex administration during emergence from anesthesia that was induced by the sugammadex-rocuronium complex, rather than by sugammadex alone.
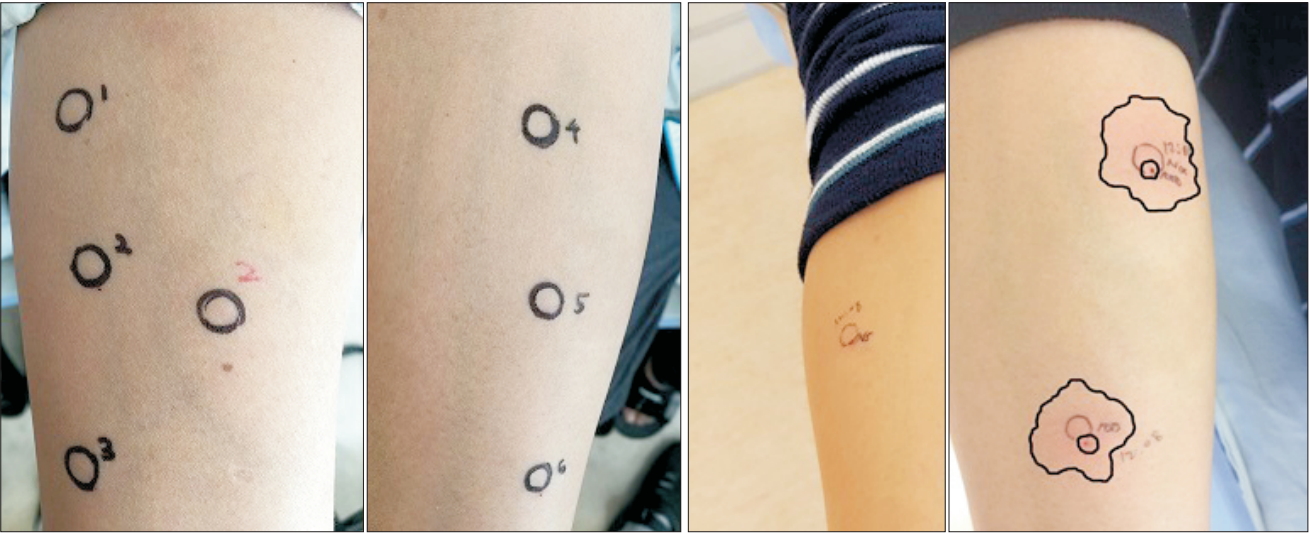
A 42-year-old man (weight: 78 kg, height: 175 cm) underwent an elective functional endoscopic sinus surgery and revision of septoplasty under general anesthesia. He had well-controlled diabetes mellitus and reported no allergies except to cat hair. He had undergone general anesthesia 10 years prior, but it was uneventful. Preoperative laboratory findings, chest radiography, electrocardiography, and pulmonary function tests were normal. The patient received 1.5 mg/kg of propofol for anesthesia induction and 0.5 mg/kg of rocuronium bromide to facilitate tracheal intubation. Anesthesia was maintained with 1.5ŌĆō2.0 vol% sevoflurane and target-controlled infusion of remifentanil. During the surgery, the patient was breathing over the ventilator due to surgical stimulation, additional 0.13 mg/kg of rocuronium was administered. One ╬╝g/kg of palonosetron and 0.4 mg/kg of ketorolac were administered intravenously 10 minutes before the surgery finished. The surgery ended in 55 minutes and 2.5 mg/kg of sugammadex was administered to reverse the neuromuscular blockade. The interval time between administration of rocuronium and sugammadex was 50 minutes. Five minutes after sugammadex administration, we confirmed that the patient could lift his head and sustain hand grasp over 5 seconds with adequate spontaneous breathing over 6 L/min, so we decided to extubate a tracheal tube. Last blood pressure was 86/48 mmHg and heart rate was 123 beats/min. Then he was transferred to the postanesthesia care unit (PACU). However, on arrival at the PACU, the patient exhibited redness in the anterior chest, swollen eyes, facial edema, and wheezing sounds in both lungs. Additionally, he complained of anxiety with dyspnea and dizziness. His blood pressure checked right after arriving PACU was 31/19 mmHg and heart rate was 130 beats/min. Under suspicion of an anaphylactic reaction, the patient was treated with rapid intravenous crystalloid and colloid fluid-loading and intravenous phenylephrine (1.3 ╬╝g/kg bolus, 500 ╬╝g cumulative dose). His blood pressure remained 56/44 mmHg; thus, we used intravenous epinephrine (0.25 ╬╝g/kg bolus, 140 ╬╝g cumulative dose), 60 ╬╝g/kg of dexamethasone, and 50 ╬╝g/kg of chlorpheniramine. Immediate laboratory tests showed that his blood glucose level was 186 mg/dl; arterial blood-gas analysis revealed PaO2 of 81 mmHg and PaCO2 of 40.9 mmHg. After 80 minutes of treatment in the PACU, his blood pressure recovered to 114/76 mmHg. Bilateral wheezing sounds and facial edema gradually improved; he was then transferred to the general ward (Fig. 1). Laboratory tests two hours after the event showed that serum tryptase and total IgE levels were significantly increased (46.9 ng/ml and 1632 IU/ml; normal ranges < 11.5 ng/ml and 0ŌĆō100 IU/ml, respectively). On the basis of clinical presentation and laboratory tests, we diagnosed anaphylactic shock due to an agent used during the surgery. To identify the causative agent, intradermal skin tests were performed four days later with all agents that had been used during the surgery: rocuronium, propofol, palonosetron, ketorolac, and sugammadex. However, all agents showed negative results (Fig. 2). The patient was discharged without any complications. One month later, he visited the Department of Allergy Medicine; the allergist strongly suggested that the anaphylactic shock after the surgery might have been triggered by sugammadex administration. However, sugammadex was not a causative agent of the anaphylactic shock, according to the previous intradermal skin test results. Thus, we suspected that the sugammadex-rocuronium complex might have led the patient to experience anaphylactic shock; we prepared intradermal tests with a sugammadex (100 mg/ml) and rocuronium (10 mg/ml) mixture at a 1 : 1 volume ratio to make complexes of sugammadex-encapsulated rocuronium. The patient underwent intradermal skin tests with 1 : 1000 and 1 : 100 diluted solutions of the sugammadex-rocuronium mixture. He showed a positive reaction to both test solutions: 8-mm wheal and 32 ├Ś 30-mm flare to the 1 : 100 solution; 7-mm wheal and 28 ├Ś 26-mm flare size to the 1 : 1000 solution (Fig. 2). We concluded that the sugammadex-rocuronium complex was the causative agent of anaphylaxis for this patient. Authors took an informed consent from the patient.
The reported incidence of sugammadex-induced anaphylaxis has varied from 1/3,000 to 1/20,000 [2,3]. However, most reports regarding sugammadex-induced anaphylaxis have indicated that sugammadex was the causative agent; there have been few reports of anaphylaxis induced by the sugammadex-rocuronium complex [4,5]. To our knowledge, this is the first case report of sugammadex-rocuronium complex-induced anaphylaxis in South Korea.
Regardless of its causative agent, when anaphylaxis occurs, prompt acknowledgement and management by an anesthesiologist is essential. A patient experiencing anaphylactic shock should be treated with rapid and specific management, including intravenous fluid replacement and the use of cardiovascular drugs. The drug of choice for anaphylactic shock is intravenous epinephrine [6]. In the present case, phenylephrine was initially administered, before the usage of epinephrine. Although phenylephrine or ephedrine can be used in cases of anaphylactic shock [7], it was ineffective at an early point in the present case. Thus, we administered intravenous epinephrine and the patient recovered from anaphylactic shock.
According to the 2012 World Allergy Organization criteria for anaphylaxis, a diagnosis of anaphylactic shock should be made on the basis of a patientŌĆÖs clinical presentation [6]. Laboratory tests, including elevated serum tryptase and serum IgE levels, are also useful in diagnosis [2]. To determine the causative agent of drug hypersensitivity, the intradermal skin test (or skin prick test) is the gold standard, but it poorly reflects procedures and test concentrations for most drugs [8]. Concerning the optimal drug dilution ratio, a solution of 1 : 100 (or more dilute) sugammadex is acceptable for performing skin tests to avoid a false-positive reaction [9]. To make a sugammadex-rocuronium mixture for use in a skin test, there is insufficient information regarding the appropriate volumetric ratio to mix the two agents in order to properly form the sugammadex-rocuronium complex. Sadleir et al. [9] and Ho et al. [4] have reported the use of sugammadex and rocuronium at a 1 : 10 volume ratio, whereas Nakanishi et al. [10] and Yamaoka et al. [5] used a 1 : 1 volume ratio. In the present case, we used a solution with a 1 : 1 volume ratio of sugammadex and rocuronium. Although the solution with a 1 : 1 volume ratio may include more free sugammadex molecules than the solution with a 1 : 10 volume ratio, we suspected that these molecules would exhibit a minimal effect because of the previous negative skin test reaction to sugammadex alone. The ideal interval between the onset of anaphylaxis and performing the hypersensitivity skin test is controversial. However, it is unknown whether reactivity to the test might be increased or decreased when the test is performed within a few days after the onset of anaphylaxis. Thus, the test is recommended after a minimum of three weeks and no more than three months after anaphylaxis [8]. However, in the present case, the intradermal skin test was performed four days after anaphylaxis because the patient requested a confirmatory test before he was discharged from the hospital.
Quantitative neuromuscular monitoring is essential for all patients receiving neuromuscular blocking agents, and there is a guideline for the dosage of sugammadex under train-of-four (TOF) monitoring, to achieve sufficient reversal of neuromuscular functions [11]. However, in the present case, we could not use the neuromuscular monitor due to a shortage of monitoring devices and rapid surgical turnover intervals; thus, we assessed neuromuscular reversal with clinical signs. In the present case, at the time of sugammadex administration, the patient was able to breathe on his own, at a rate of > 5 L/min; we assumed that the patientŌĆÖs TOF count was approximately 2 to 4, based on the time of the last dose of rocuronium, as well as his clinical signs. Under the guideline described above, we administered 2.5 mg/kg of sugammadex to the patient.
Because the clinical features of the present case were very similar to anaphylaxis induced by sugammadex alone [12] and the onset occurred immediately after sugammadex administration, we suspected that the causative agent might be sugammadex. Thus, we initially regarded the negative result in the postoperative intradermal test of sugammadex as a false-negative finding. Later, we were reminded that sugammadex encapsulates rocuronium at a 1 : 1 molecular ratio, thus inactivating the neuromuscular blockade; we investigated whether this sugammadex-rocuronium complex might act as a separate antigen for the patient. We ultimately concluded that the complex was the causative agent for anaphylactic shock in this patient.
Sugammadex is a ╬│-cyclodextrin derivative; thus, it is shaped like a truncated cone with a hydrophobic cavity. The cavity of sugammadex encapsulates the steroid backbone of rocuronium with very high affinity, thereby forming the sugammadex-rocuronium complex. The properties of the sugammadex-rocuronium complex are not yet well-established; however, changes have been reported in drug solubility, delivery, distribution, or stability as a result of the formation of the complex. These suggest potential structural and chemical alterations to both rocuronium and sugammadex. Notably, such changes may create sugammadex-rocuronium compound antigens and cause allergic reactions, although such reactions may not occur in the presence of sugammadex or rocuronium alone [13]. Although there have been few reports of sugammadex-rocuronium complex-induced anaphylaxis [4,5] and exact mechanisms of hypersensitivity reactions are not fully known, anesthesiologists and healthcare providers should be aware of the possibility of anaphylaxis from the sugammadex-rocuronium complex, as well as from sugammadex or rocuronium alone.
Fig.┬Ā1.
Trend chart shows vital signs of the patient between the time of sugammadex administration and transfer to the general ward. Marks above the chart indicate major events and drugs administered to the patient, corresponding to points within the timeline (S: administration of sugammadex, X: exit from the operating room, R: arrival at the PACU, Ō¢Į: administration of 1.3 ┬Ąg/kg of intravenous phenylephrine, ŌŚŗ: administration of 60 ┬Ąg/kg of intravenous dexamethasone, Ōåō: administration of 0.25 ┬Ąg/kg of intravenous epinephrine, P: administration of 50 ┬Ąg/kg of intravenous chlorpheniramine, W: transfer to general ward).
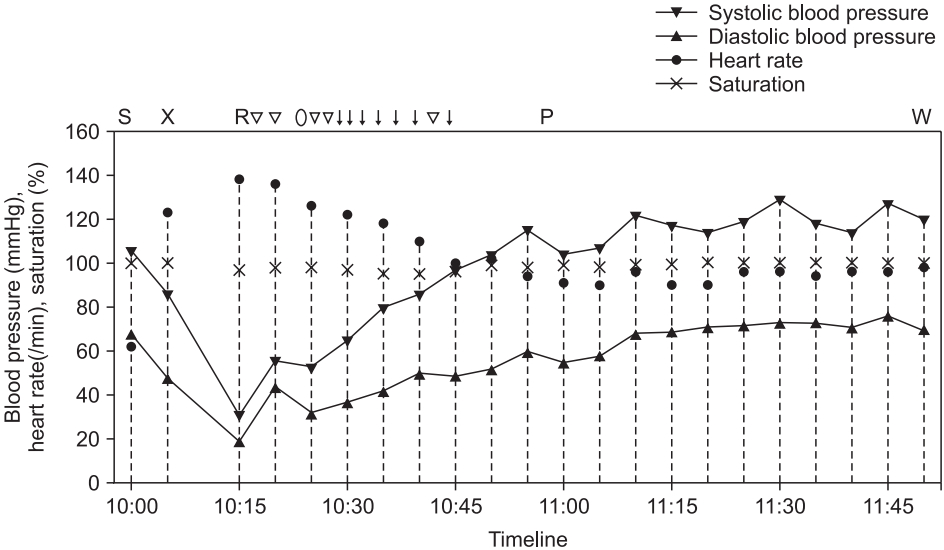
Fig.┬Ā2.
Results of intradermal skin tests on volar area of forearm. Two photos on the left side show negative results of tests performed with agents used during anesthesia, four days after the event. All agents were diluted at a 1 : 1000 ratio. Sugammadex alone was tested in both 1 : 1000 and 1 : 100 solutions (Number 1: propofol; number 2: sugammadex; number 2 with red color: 1 : 100 diluted solution of sugammadex; number 3: palonosetron; number 4: rocuronium; number 5: ketorolac; number 6: saline for negative control). Two photos on the right side show results of tests with sugammadex-rocuronium complex, one month after the event. 1 : 100 diluted solution shown at the bottom/center (8-mm wheal size, 32 ├Ś 30-mm flare size), 1 : 1000 diluted solution shown at the right upper-center (7-mm wheal size, 28 ├Ś 26-mm flare size), and saline for negative control shown at the left.
References
1. Godai K, Hasegawa-Moriyama M, Kuniyoshi T, Kakoi T, Ikoma K, Isowaki S, et al. Three cases of suspected sugammadex-induced hypersensitivity reactions. Br J Anaesth 2012; 109: 216-8.



2. Takazawa T, Mitsuhata H, Mertes PM. Sugammadex and rocuronium-induced anaphylaxis. J Anesth 2016; 30: 290-7.



3. Min KC, Woo T, Assaid C, McCrea J, Gurner DM, Sisk CM, et al. Incidence of hypersensitivity and anaphylaxis with sugammadex. J Clin Anesth 2018; 47: 67-73.


4. Ho G, Clarke RC, Sadleir PH, Platt PR. The first case report of anaphylaxis caused by the inclusion complex of rocuronium and sugammadex. A A Case Rep 2016; 7: 190-2.


5. Yamaoka M, Deguchi M, Ninomiya K, Kurasako T, Matsumoto M. A suspected case of rocuronium-sugammadex complex-induced anaphylactic shock after cesarean section. J Anesth 2017; 31: 148-51.



6. Simons FE, Ardusso LR, Dimov V, Ebisawa M, El-Gamal YM, et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol 2012; 12: 389-99.


7. Hwang MH, Won YJ, Lee IO, Koo EH, Jung WJ. A suspected case of sugammadex-induced anaphylactic shock: A case report. Anesth Pain Med 2015; 10: 288-90.


8. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002; 57: 45-51.

9. Sadleir PH, Russell T, Clarke RC, Maycock E, Platt PR. Intraoperative anaphylaxis to sugammadex and a protocol for intradermal skin testing. Anaesth Intensive Care 2014; 42: 93-6.


10. Nakanishi T, Ishida K, Utada K, Yamaguchi M, Matsumoto M. Anaphylaxis to sugammadex diagnosed by skin prick testing using both sugammadex and a sugammadex-rocuronium mixture. Anaesth Intensive Care 2016; 44: 122-4.

11. Brull SJ, Kopman AF. Current status of neuromuscular reversal and monitoring: challenges and opportunities. Anesthesiology 2017; 126: 173-90.











