 |
 |
|
|
Abstract
Background
Methods
Results
Acknowledgments
References
Fig. 1
Simulation based on the Schnider model using infusion history data for propofol administered via target effect-site concentration (Ce)-controlled infusion based on the modified Marsh model in normal-weight (upper panel) and underweight (lower panel) patients. Dotted and solid lines indicate the target Ce values of the infusion using the modified Marsh model and the predicted Ce values in a simulation based on the Schnider model, respectively. BMI: body mass index.
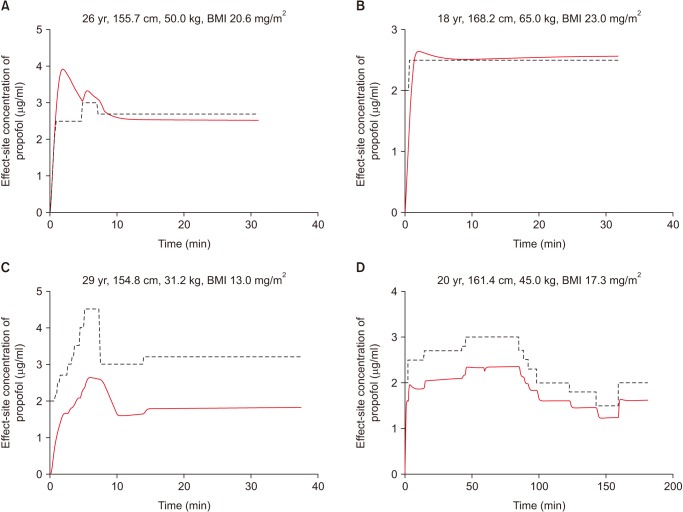
Fig. 2
Simulated clearance calculated by the modified Marsh (dotted line) and Schnider (solid line) models relative to the change in body weight of a 40-year-old female participant who is 165 cm tall. BMI: body mass index.
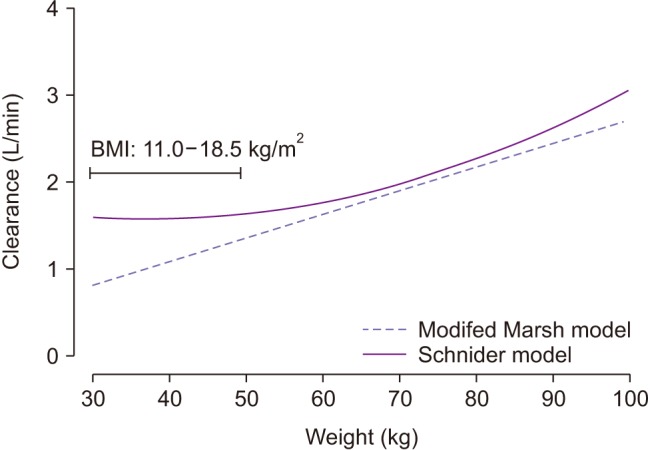
Fig. 3
Simulation showing the effects of height, sex, age, and weight on clearance in the Schnider model. (A) Male participants are 35 years old. (B) Participants are 35 years old and 165 cm tall. (C) Male participants are 165 cm tall. (D) Male participants are 35 years old.
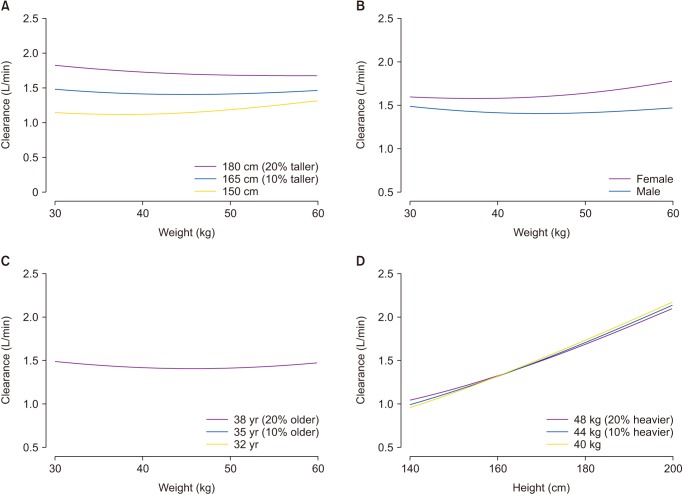
Table 1
Pharmacokinetic Parameters and Blood-brain Equilibration Rate Constant (ke0) Used in the Modified Marsh and Schnider Model
V1: central volume of distribution, k: microrate constant, V2: rapid peripheral volume of distribution, V3: slow peripheral volume of distribution, Cl: metabolic clearance, Q1: inter-compartmental clearance between central and rapid peripheral compartments, Q2: inter-compartmental clearance between central and slow peripheral compartments, ke0: blood-brain equilibration rate constant, LBM: lean body mass.
Table 2
Characteristics of the Patients and Dosages of Anesthetic Agents Administered during Surgery
Data are expressed as the means ± SD, median (25–75%), or numbers, as appropriate. Patient characteristics are compared using the two-sample t-test, Mann–Whitney rank sum test, or chi-square test, as appropriate. ASA PS: American Society of Anesthesiologists Physical Status, BMI: body mass index. *P < 0.05 vs. normal-weight patients, †dose administered during the entire anesthetic period.









