Comparing epidural surgical anesthesia and spinal anesthesia following epidural labor analgesia for intrapartum cesarean section: a prospective randomized controlled trial
Article information
Abstract
Background
The conversion of epidural labor analgesia (ELA) to epidural surgical anesthesia (ESA) for intrapartum cesarean section (CS) often fails, resulting in intraoperative pain. Spinal anesthesia (SA) can provide a denser sensory block than ESA. The purpose of this prospective, non-blinded, parallel-arm, randomized trial was to compare the rate of pain-free surgery between ESA and SA following ELA for intrapartum CS.
Methods
Both groups received continuous epidural infusions for labor pain at a rate of 10 ml/h. In the ESA group (n = 163), ESA was performed with 17 ml of 2% lidocaine mixed with 100 µg fentanyl, 1 : 200,000 epinephrine, and 2 mEq bicarbonate. In the SA group (n = 160), SA was induced with 10 mg of 0.5% hyperbaric bupivacaine and 15 µg fentanyl. We investigated the failure rate of achieving pain-free surgery and the incidence of complications between the two groups.
Results
The failure rate of achieving pain-free surgery was higher in the ESA group than the SA group (15.3% vs. 2.5%, P < 0.001). There was no statistical difference between the two groups in the rate of conversion to general anesthesia; however, the rate of analgesic requirement was higher in the ESA group than in the SA group (12.9% vs. 1.3%, P < 0.001). The incidence of high block, nausea, vomiting, hypotension, and shivering and Apgar scores were comparable between the two groups.
Conclusions
SA after ELA can lower the failure rate of pain-free surgery during intrapartum CS compared to ESA after ELA.
Introduction
Well-functioning epidural labor analgesia (ELA) can be extended for use as epidural surgical anesthesia (ESA) for intrapartum cesarean section (CS). ESA using the epidural catheter for ELA might be a reliable and effective anesthetic method for emergency CS [1]. However, ESA for CS has been associated with unsatisfactory outcomes, including conversion to another anesthetic method or failure to achieve a satisfactory block (1.7–38%) [1234]. General anesthesia (GA) or spinal anesthesia (SA) after failure of ESA have potential problems such as airway difficulty or high SA [56].
In contrast, the GA conversion rate and complications of SA after ELA without attempting to perform ESA were comparable to cases only using SA without ELA [7]. In addition, SA after ELA may be preferred given its rapid induction and adequate muscle relaxation [8]. However, previous studies that compared the rates of failure and side effects between ESA and SA after ELA were retrospective cohort studies.
In this study, we investigated the rate of pain-free intrapartum CS and the incidence of complications to compare ESA and SA following ELA in a prospective randomized manner.
Materials and Methods
This study was approved by the Ethics and Research Committee of Cheil general hospital and is registered at cris.nih.go.kr. This prospective, randomized, non-blinded study included participants who were scheduled for intrapartum CS with ELA. We obtained written informed consent from all patients included in the study's prospective sample between July 1, 2014 and September 6, 2015. Patients had an American Society of Anesthesiologists physical status of I–II; full-term, singleton pregnancy; and urgency classification category 3 (needing early delivery but no maternal or fetal compromise) by the Royal College of Anaesthetists [9]. The patients had fasted for at least 8 hours prior to surgery. Exclusion criteria were as follows: malfunctioning epidural catheter or improper epidural placement, less than 2-h interval between epidural analgesia top-up and CS, complicated pregnancy (such as multiple gestation, placenta previa, and pregnancy-induced hypertension), antepartum hemorrhage, cardiac disease, contraindication to SA, or risk of difficult airway or aspiration (including maternal obesity with body mass index ≥ 30 kg/m2, symptoms of gastroesophageal reflux, intestinal obstruction, ileus, elevated intracranial pressure, neuromuscular disease, mouth opening less than 4 cm, history of difficult intubation, or Mallampati classification Class III or Class IV) [10]. Severity of labor pain was evaluated with a numerical rating scale (NRS: 0–10). Malfunctioning epidural catheter or improper epidural placement included unsatisfactory analgesia (NRS > 3, more than 2 additional epidural boluses), manipulation or replacement of the epidural catheter, unilateral blockade, catheter occlusion, or catheter migration (intravascular, subarachnoid).
Prior to performing ELA, 500 ml of Ringer's lactate solution was administered intravenously in the delivery room. According to routine protocols, non-invasive arterial blood pressure was measured every 5 minutes. With patients in the lateral decubitus position, lidocaine was infiltrated into the subcutaneous tissue in the L3–4 or L4–5 intervertebral space. An 18-gauge Tuohy needle (Portex® Epidural Minipack, SIMS Portex Ltd., Hythe, Kent, UK) was inserted using the midline approach until the operator felt the needle enter the interspinous ligament. The needle was advanced until the practitioner felt a loss of resistance using a syringe filled with air. The epidural catheter was threaded if no cerebrospinal fluid (CSF) leaked and no paresthesia was noted. A 20-gauge multi-orifice epidural catheter (Portex® Epidural Minipack, SIMS Portex Ltd., Hythe, Kent, UK) was inserted 4–5 cm into the epidural space. After aspiration, a 3 ml test dose of 2% lidocaine, followed by an 8 ml dose of 0.2% ropivacaine with 50 µg of fentanyl were administered via the epidural catheter. Continuous epidural analgesia was initiated at 10 ml/h using 0.1% ropivacaine with 1.5 µg/ml of fentanyl. Breakthrough pain, defined as labor pain of NRS > 3, was managed with epidural boluses of 8–10 ml ropivacaine (0.2%).
Prior to entering the operating room for CS, each participant filled out a detailed informed-consent form in the waiting room. They were randomized to receive either ESA after ELA (the ESA group) or SA after ELA (the SA group). Randomization was performed according to computer-generated codes contained in opaque, sealed, and sequentially numbered envelopes. Participants in both groups were monitored with automated blood pressure cuffs, electrocardiograms, and pulse oximetry after arriving in the operating room. After resting undisturbed in the supine position for several minutes, noninvasive blood pressure was measured three times. The baseline systolic blood pressure (SBP) was calculated as the mean value of three recordings. Hypotension was defined as a 20% decrease from the baseline SBP.
We assessed the pre-existing sensory block level with a pinprick using a 24-gauge needle and a cotton swab doused with alcohol. Sensation was tested from the lower abdomen upward. After anesthetic induction, the motor block was assessed using the modified Bromage scale (BS 0 = able to raise extended leg; BS 1 = able to flex knee only; BS 2 = able to flex ankle only; BS 3 = unable to move lower limbs at all) at 5 minute intervals.
All anesthesia procedures were performed by residents (in their third year, or more senior) or staff anesthesiologists with experience performing > 500 cases of neuraxial anesthesia for CS. In the ESA group, a 24.1 ml mixed solution of lidocaine (20 ml of 2% lidocaine mixed with 100 µg fentanyl, 1 : 200,000 epinephrine, and 2 mEq sodium bicarbonate) was prepared immediately before administration. After negative aspiration from the epidural catheter, 17 ml of the mixed solution of lidocaine was injected epidurally over 3 min. Five minutes after the injection was completed, the block level was assessed every minute using a cotton swab doused with alcohol in order to determine if a bilateral block along the mid-clavicular line up to T5 was achieved. If this block was not achieved within 20 minutes of the injection, 5 ml boluses of the same solution were injected through the catheter. If the sensory block for coldness was still absent at the T5 level 30 min after the supplemental dose, the top-up was considered a failure.
In the SA group, the epidural catheter for ELA was removed before the SA procedure. With the patient in the lateral decubitus position, a 25-gauge pencil point spinal needle (Pencan, B.Braun, Melsungen, Germany) was placed at the L3-4 interspace, while Ringer's lactate solution was administered as a rapid crystalloid co-load. After observing free flowing CSF, 10 mg of 0.5% hyperbaric bupivacaine plus 15 µg fentanyl was administered into the subarachnoid space. If we could not identify free flowing CSF on repeated attempts, SA was converted into general anesthesia (GA) for CS. After SA, phenylephrine was infused continuously at 50 µg/min in order to prevent hypotension. The patient's blood pressure and heart rate were measured every minute until delivery and every 5 minutes thereafter. Phenylephrine infusion continued as long as the SBP was below baseline SBP and was stopped if it exceeded the baseline SBP. Hypotension that occurred despite phenylephrine infusion was treated with a bolus of 100 µg phenylephrine if the patient's HR was ≥ 60 bpm. In contrast, if the HR was < 60 bpm, then a bolus of 10 mg ephedrine was administered [11].
A skin incision was permitted when there was an adequate loss of sensation to cold block at T5 and patients experienced no pain from a skin pinch (using forceps) at the surgical site. A failed sensory block was defined as no sensory block after SA in the SA group or no change at the level of the sensory block after augmentation of the epidural analgesia, in comparison to a preexisting sensory block produced by ELA in the ESA group. A high neuraxial block was defined as dyspnea with sensory and motor block of the upper extremity after epidural augmentation or spinal injection [12]. Failure of pain-free surgery was divided into two categories: conversion to GA and analgesic supplements. We converted neuraxial anesthesia to GA in both groups in the following situations: failed sensory block, the upper level of sensory block to coldness below T5, or patchy block or pain from forceps pinching at the surgical site in patients whose upper level of sensory block to coldness was equal or above T5. We evaluated intraoperative pain using 100-mm visual analogue scales (VAS). If a VAS ≥ 30 mm was recorded, 100 µg fentanyl was injected intravenously as a rescue analgesic. If the pain was not managed with intravenous fentanyl, the anesthesia was considered to be of poor quality, and GA was induced. General anesthesia was induced through tracheal intubation with thiopental (5 mg/kg), suxamethonium (1.5 mg/kg), and labetalol (10 mg) and maintained with sevoflurane and N2O. If the patient received GA after ineffective 100 µg fentanyl, the case was regarded as a conversion to GA due to poor analgesia quality. Apgar scores (1 and 5 min) and birth weight were recorded after delivery. Adverse effects such as nausea, vomiting, and shivering after anesthesia induction were monitored throughout the operation.
The primary outcome of this study was a comparison of the rates of pain-free surgery. Kinsella [1] reported that the rate of failure to achieve a pain-free operation following ELA was 24%. A sample size of 157 in each group was needed to detect a 50% reduction in the incidence of failure of pain-free surgery (power = 0.8, α = 0.05) from 24% to 12%. Therefore, a total of 350 participants were examined to compensate for a 10% dropout rate. Data were expressed as mean ± SD, median (interquartile range), or number (%). Data were compared using independent t tests, Mann-Whitney U tests, or χ2 analysis. Statistical analyses, including sample size and power calculations, were performed with SigmaStat version 4.0 (San Jose, CA, USA). P values < 0.05 were considered significant.
Results
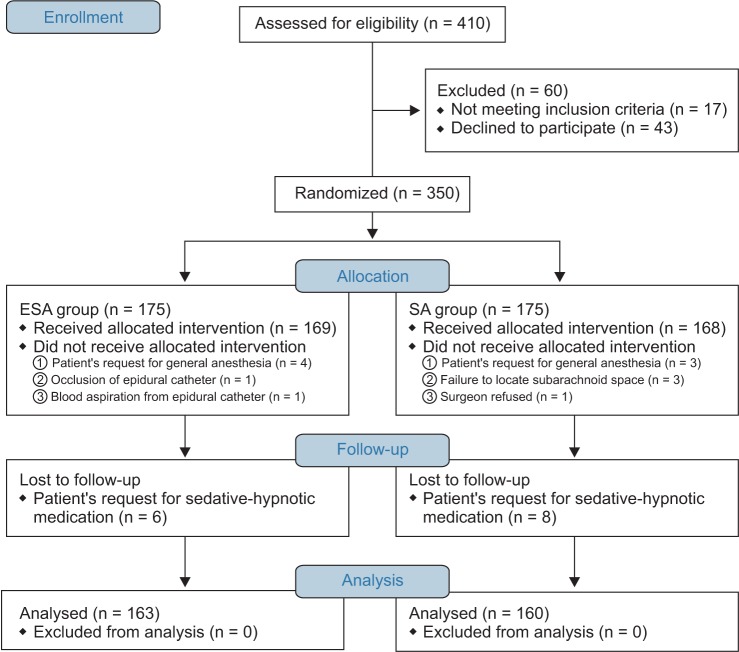
Of the 410 participants considered for eligibility, 60 were excluded (exclusion criteria met, n = 17; refusal to participate, n = 43). Consent was obtained from the remaining 350 participants who were then randomized. Twelve participants in the ESA group and 15 in the SA group dropped out (Fig. 1).
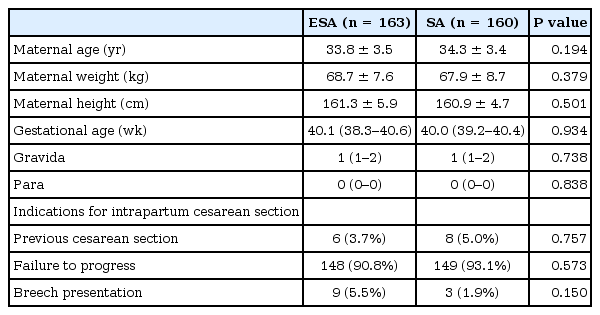
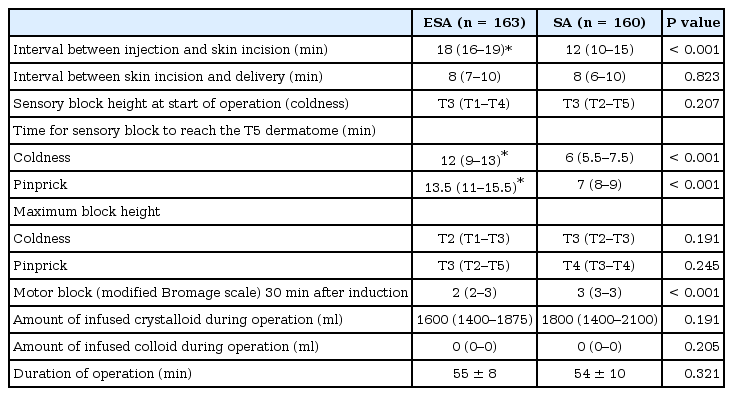
There were no significant differences in the baseline characteristics or the indications for intrapartum CS between the two groups including maternal age, weight, height, gestational age, gravida, or para (Table 1). Characteristics and analgesic quality of ELA before CS are outlined in Table 2. There were no significant differences between the two groups. The clinical characteristics related to anesthesia and the surgeries performed in the two groups are summarized in Table 3. The two groups were also similar with regard to the time interval between skin incision and delivery, sensory block heights at the start of the operation (coldness), maximum block heights (coldness and pinprick), the amount of infused crystalloid and colloid during the surgery, and duration of the operation. There were statistically significant differences between the two groups with regard to the time interval between injection and skin incision, time for the sensory block to reach the T5 dermatome (coldness and pinprick), and the motor block evaluated by the modified Bromage scale 30 min after induction (Table 3).
The failure rate of pain-free surgery was higher in the ESA group than in the SA group (15.3% vs. 2.5%, P < 0.001). The rate of conversion to GA was not different between the two groups. However, the rate of use of analgesic supplements was markedly greater in the ESA group than in the SA group (12.9% vs. 1.3%, P < 0.001). The phenylephrine requirement was greater in the SA group than in the ESA group (P < 0.001). Intra-operative pain developed in 23 patients in the ESA group. Of these, the pain in 21 patients was controlled with 100 µg fentanyl. Two patients were classified with poor anesthesia quality and were converted to GA. The incidence of high spinal block, nausea, vomiting, hypotension, and shivering and Apgar scores were comparable between the two groups (Table 4). One patient in the SA group had a high neuraxial block, but she did not require respiratory support or intubation. This particular patient did not receive rescue epidural top-up in the delivery room.
Discussion
In this prospective randomized study, the failure rate of pain-free surgery was higher in the ESA group than in the SA group. Although the analgesic requirement was significantly higher in the ESA group than in the SA group (due to a higher incidence of intraoperative pain in the ESA group), the rate of conversion to GA was comparable between the two groups. To the best of our knowledge, this study is the first prospective randomized study that compared ESA and SA after ELA during intrapartum CS.
The prospective study of 5,080 emergency CS cases by Kinsella [1] found that the failure rate of pain-free surgery in ESA after ELA was 24%. The rate of failure to achieve a pain-free surgery after achieving ESA (not conversion to another anesthesia, including epidural site leakage and sensory block after test dose injection) following ELA was 22.8% [1]. This rate was slightly higher than the failure rate of pain-free surgery in our study (15.3%). The difference may have resulted from varying degrees of urgency for CS and less frequent use of fentanyl and epinephrine in the epidural solution in the Kinsella study compared to our study [11314].
Based on several studies with varying degrees of urgency for CS, the conversion rate from ESA (after ELA) to GA is 5.0–10.2% [1715]. This GA conversion rate from ESA could depend on the addition of lipophilic opioid and epinephrine in the epidural mixtures and the degree of urgency for CS [1131415]. Lipophilic opioid and epinephrine in the epidural solution may reduce this rate, while a high degree of urgency (urgency category by the Royal College of Anaesthetists 1 > 2 > 3) could increase this rate [19131415].
In the prospective, non-randomized study by Kinsella [1], the conversion rate from ESA to GA was 5.6% if a diverse degree of emergency was included, but this rate decreased to 2.4% if restricted to urgency category 3, which is similar to our result (2.5%). In the study by Kinsella [1], CS was initiated in 27% of patients with inadequate blocks. However, we converted to GA if coldness was not attained at the T5 level. This protocol may have increased the rate of GA in our study. In contrast, inclusion of patients who received ESA with epidural solutions not containing lipophilic opioids and epinephrine in the study by Kinsella may have increased the GA conversion rate of ESA [11314].
The GA conversion rate for ESA after ELA in a retrospective cohort study by Huang et al. [8] was much higher than that of our study (10.2% vs. 2.5%). The higher failure rate of ESA in Huang et al.'s study may be related to the higher degree of emergency and the absence of lipophilic opioids and epinephrine in the epidural solution [1314].
There are very few studies regarding SA after ELA because ESA using an indwelling epidural catheter for ELA may be the preferred anesthetic method [1]. We only found two studies that compared SA and ESA following ELA for emergency CS. These were retrospective cohort studies by Visser et al. [7] and Huang et al. [8].
The conversion rates to GA from SA after ELA were 3.9% and 13.7% in the studies by Visser et al. [7] and Huang et al. [8], respectively, which were both higher than that of this study (1.3%). The higher rates in the two studies could be related to the absence of lipophilic opioid and the higher degree of urgency even though the study by Huang et al. did not address the detailed indications for GA use [11617].
Parturients undergoing intrapartum CS may experience more severe anxiety intraoperatively than those undergoing elective CS under regional anesthesia. Increased anxiety could exacerbate intraoperative pain and enhance anesthetic demand [1718]. In addition, tissue injury and pain experienced as labor progresses could decrease the threshold of pain perception in intrapartum CS by the mechanism of central sensitization [1819]. Therefore, SA with a dense sensory block may be more effective at suppressing intraoperative pain than ESA during intrapartum CS [120].
High blocks requiring respiratory support were reported during SA after ELA [2122]. This complication was mainly associated with SA after ESA failure; however, it rarely developed in patients receiving SA with ELA not topped up for ESA or those receiving rescue epidural bolus during ELA [17].
The findings of this study have some limitations. First, this study only included patients with urgency classification category 3, so our results cannot be extrapolated to patients in all urgency classification categories undergoing intrapartum CS. For patients in higher urgency categories than the inclusion criteria of our study, the decision to delivery interval could be more critical for maternal and neonatal outcomes [23]. In these cases, effective anesthesia without delaying the decision to delivery interval is recommended. In this study, we examined the interval between injection of drugs and start of the operation. Not surprisingly, this time interval was shorter in the SA group. However, ESA after ELA could have an advantage over SA after ELA if it is performed outside the operating room in terms of no delay in the decision to delivery interval. In addition, attempting SA in patients after removing a well-functioning epidural catheter may be time consuming and cause discomfort, especially in patients with labor pain. In other aspects, early injection of local anesthetics into the indwelling epidural catheter outside the operating room can have potential problems such as an increased anesthetic workload, difficulty in patient monitoring, and risks to patient safety. Therefore, the appropriate choice of anesthetic method should be based on the available resources and individual situation. The risk factors of failed conversion of ELA to ESA (other than a high degree of emergency and no addition of opioid and epinephrine in the epidural solution) include an increasing number of rescue epidural boluses during ELA and care by a non-obstetric, anesthesiologist [15]. Therefore, especially under circumstances involving risk factors of ESA failure, the conversion to SA (instead of ELA augmentation) may be recommended to achieve pain-free CS.
Second, it is difficult to determine whether equipotent local anesthetics were used in both groups in this study. To compare the analgesic effects of local anesthetics in both groups, injection of equipotent local anesthetics could be recommended. However, comparing the potency of different local anesthetics with different routes of administration may be difficult. Therefore, we administered local anesthetics and additives with fast onset and good analgesic effect in this study [24].
In conclusion, SA may lower the failure rate of pain-free surgery, as well as the rescue analgesic requirement during intrapartum CS compared with ESA.