Introduction
It is common for the core body temperature to drop below 35℃ within the first 40 min of administration of anesthesia to a patient. If the body temperature is not controlled intraoperatively, patients are likely to suffer postoperative hypothermia [
1]. Perioperative hypothermia is a recognized and common side effect of anesthesia that inhibits normal thermoregulation. It frequently prolongs the duration of action of both anesthetics and neuromuscular blocking agents; delays postoperative recovery; and increases the incidence of surgical wound infections, coagulopathy, and cardiac events [
2]. Billeter et al. [
3] reported that patients with postoperative hypothermia had a 4-fold increase in mortality as well as a 2-fold increase in the complication rate when compared to patients with postoperative normothermia. Therefore, the National Institute for Health and Clinical Excellence released guidelines for the management of inadvertent perioperative hypothermia in adults [
1]. These guidelines recommend that intravenous fluids with volumes > 500 ml should be warmed to 37℃ using a fluid warming device, as infusion of 1 L of an intravenous fluid at room temperature (21℃) could reduce core body temperature by 0.25℃/ L [
1,
4,
5]. Accordingly, various fluid warming devices designed to deliver fluid at a temperature of approximately 37℃ have been developed. The use of perioperative warming devices has now become routine [
6], and many studies have shown that maintaining perioperative normothermia with preventive treatment and aggressive use of warming devices is helpful for reducing morbidity and complications [
3,
7,
8,
9].
The standard-flow disposable fluid warming set 3M™ Ranger™ Blood/ Fluid Warming System (Standard Ranger, Arizant Healthcare, Inc., MN, USA) uses \countercurrent metal technology for fluid warming and has been shown to significantly increase the delivered fluid temperature by increasing the infusion rate to 1 L/hr [
10,
11]. The Mega Acer Kit® (MAK, Ace Medical, Seoul, Korea) is a recently developed and novel fluid warming system that is able to warm fluid via the lumen of a newly designed heated and humidified breathing circuit (HHBC) inclusive of a fluid-warming unit. Therefore, we hypothesized that the MAK would be more effective at maintaining distal esophageal temperature than the Standard Ranger during anesthesia. However, the clinical and laboratory efficacy of these two systems have not yet been compared.
The present study aimed to compare the fluid warming performances and the efficacy of hypothermia prevention of the MAK and the Standard Ranger in patients undergoing spinal surgery under general anesthesia.
Materials and Methods
This prospective, randomized study was approved by our Institutional Review Board and written and informed consent was obtained from all patients. We enrolled patients classified by the American Society of Anesthesiologists as physical status I or II, who were aged between 20 and 65 years and scheduled for elective spinal surgery with duration of at least 180 min between October 2011 and March 2012. Patients with preoperative hypothermia (< 36.0℃) or hyperthermia (> 38℃), thyroid diseases, diabetes, hypertension; patients receiving medicines that could affect thermoregulation; and patients undergoing emergency surgery were excluded from the study.
Patients were randomized by means of a random number table to receive intravenous fluids either without the use of warming devices (group C, n = 30), using Standard Ranger (set point: 41℃, group R, n = 30), or using MAK (set point: 38℃, group M, n = 30). Patients were administered 0.05 mg/kg of midazolam intramuscularly 30 min before total intravenous anesthesia with propofol and remifentanil, and mechanical ventilation was set to maintain 30 to 35 mmHg end-tidal carbon dioxide. For delivery of 3 L/min of 50% oxygen, a conventional HHBC (Heated Circuit Kit, Ace Medical, Seoul, South Korea) was used in groups C and R, while a new HHBC with a fluid-warming unit (Mega Acer Kit, Ace Medical, Seoul, South Korea) was used in group M. After induction, fluids that had been stored for over 24 h in the operating theatre at 22 ± 2℃ were delivered at 400 ml/h through preheated warming devices according to the manufacturer's instructions. The infusion rate was not altered unless estimated blood loss exceeded allowable blood loss; in which case the infusion rate was increased, repeated 250 ml bolus infusions of fluids were performed, or transfusions were carried out according to the patient's hemodynamic status. Affected patients were subsequently excluded from the analysis.
The temperature monitoring sites are shown in
Fig. 1. These fluid temperatures were measured using a fluid thermometer (KTH 300 I, Kimo instruments, Edenbridge, UK) with a specially designed device similar to a Y-connector with a side port through which a wire Pt 100 temperature probe (KRGA-50, Kimo instruments, Edenbridge, UK) was inserted under sterile conditions, while the remaining side ports were attached directly to the intravenous tubing. Baseline temperature values at the 3 recording points were recorded before infusing the heated fluid and measurements were taken at 15 min intervals for a total duration of 180 min. The distal esophageal temperature (Teso), room temperature, mean arterial pressure (MAP), and heart rate (HR) were recorded after induction and simultaneously with fluid temperature measurements. When Teso was < 35.0℃, a forced-air convective warming device (the upper body, Bair Hugger, Arizant Healthcare, Inc., MN, USA) was applied to maintain Teso temperature above 35.0℃.
Change in maximal declined Teso and final Teso temperatures were calculated from baseline [Δ Teso (max), Δ Teso (final)]. The expected change in mean body temperature (MBT) according to fluid temperature at the T3 recording point was calculated for each group at the 180 min time point. This was done using Horowitz's formula below:
where ΔMBT is the change in MBT, TF is the temperature of the fluid infused, TPt is the patient's baseline core temperature (℃), SF is the specific heat of the fluid infused (1.0 kcal/L/℃ for saline), Vol is the volume of the fluid infused (in L), SPt is the specific heat of human tissue (0.83 kcal/L/℃), and Wt is the weight of the patient (in kg) [
12].
The primary outcome measure of this study was the intraoperative distal esophageal temperature. Secondary outcome measures included fluid temperatures and the number of patients requiring application of the forced-air convective warming device.
As no references were available for aiding calculation of the sample size, total sample size in this study was based on the level of statistical significance defined as α = 0.05, β = 0.2, and the effect size of 0.25. A minimum of 29 patients per group was required to test our hypothesis. In order to compensate for possible dropouts (5%), 30 patients per group were enrolled in this study.
Statistical analysis was performed using the Statistical Package for the Social Sciences software package (SPSS, Version 20.0, SPSS Inc., Chicago, IL, USA). All recorded values were presented as the mean ± standard deviation (SD) or as the number of patients. The χ2 and one-way analysis of variance (ANOVA) tests were used to analyze demographic data, intraoperative physiological data, infused fluid temperature values, and esophageal temperature values; but not the time-sequence data. Continuous normally distributed fluid and esophageal temperature values were compared using repeated ANOVA and the Turkey posthoc test. A P value < 0.05 was considered statistically significant.
Results
A total of 90 patients were enrolled in the study and 1170 temperature measurements were recorded during fluid infusions. No patients were excluded from the final data analysis. No statistically significant differences in the demographic data, intraoperative blood loss, and urine output was observed among the groups (
Table 1).
Baseline Teso values were similar and tended to decrease across the 3 groups (group C: 36.3 ± 0.2℃, Group R: 36.4 ± 0.2℃, group M: 36.4 ± 0.2℃) (
Fig. 2). Teso in group M was significantly higher when compared with that in groups C and R throughout the study period (P < 0.05). The maximal declined Teso and the final Teso were significantly higher in group M (35.6 ± 0.2℃ and 35.8 ± 0.3℃, respectively) with compared with groups R (34.9 ± 0.2℃, 35.1 ± 0.1℃) and C (34.6 ± 0.3℃, 34.8 ± 0.3℃) (P < 0.05,
Table 2). The estimated change in MBT after saline infusion at a flow rate of 400 ml/h for 3 h was significantly lower in group M (0.02 ± 0.01℃) when compared with groups R (0.09 ± 0.02℃) and C (0.32 ± 0.05℃) (P < 0.05,
Table 2). The number of patients requiring application of a forced-air convective warming device was significantly lower in group M (n = 0) when compared with that in groups R (n = 17) and C (n = 30) (P < 0.01,
Table 2).
No significant difference in baseline fluid temperature before warming (TF1) was observed among the 3 groups (
Table 3). The TF2 and TF3 were significantly higher in group M when compared with those in groups C and R throughout the study period (P < 0.05,
Figs. 3A and 3B). The overall mean values of TF2 and TF3 were significantly higher in group M when compared with those in groups C and R (37.4 ± 1.7℃ and 35.4 ± 1.0℃ vs. 23.5 ± 0.3℃ and 23.0 ± 0.3℃, 34.7 ± 0.8℃ and 32.8 ± 0.6℃, respectively, P < 0.05,
Table 3).
Room temperature was maintained at 23.1 ± 0.8℃, 23.1 ± 0.6℃, and 23.2 ± 0.7℃, respectively, with no significant difference among the groups (data not shown).
In addition, no significant changes in MAP or HR were observed among the 3 groups (data not shown).
Discussion
This study showed that the MAK, a new HHBC containing a fluid warming device, was more effective than the Standard Ranger with a conventional HHBC at preventing intraoperative hypothermia (< 35.0℃) during fluid infusion at a flow rate of 400 ml/h in patients undergoing spinal surgery.
The devices that have been used in our hospital to warm intravenous fluids operate on a number of principles including dry heat, countercurrent water baths, countercurrent metal technology, or magnetic induction. The MAK consists of a fluid line (100 cm long with a volume of 5 ml) that is placed along a heating wire, which is wrapped in cotton within a humidified and heated circuit, and is equipped with a temperature controller. This system is designed to deliver warmed and humidified gas to the patient. The MAK primarily warms the fluid directly using heated convective air currents as the fluid passes through the inspiratory gas limb. In this study, we used room-temperature saline to compare the capabilities of the new MAK, which is designed to warm the fluid via the heated circuit lumen, and the Standard Ranger, which uses countercurrent metal technology. The MAK was able to maintain higher outlet and distal fluid temperatures as well as a higher intraoperative Teso at a flow rate of 400 ml/h without the use of additional body warming devices when compared to the Standard Ranger.
The ideal fluid warming device for intraoperative use would be capable of delivering 37℃ fluids over a wide range of flow rates and clinical conditions [
13,
14]. Changes in the temperature of the delivered fluid can be influenced by device-specific maximum flow rates as well as the outflow tubing length extending from the warming device to the temperature recording points [
10,
11,
12]. Therefore, choice of a fluid warming device capable of maintaining the temperature at 37℃ should be based on the device-specific maximum flow rate. Turner at al. [
11] suggested that the Bair Hugger, which claims maximal flow rates up to 1 L/h (17 ml/min), is suitable only for prolonged minor surgery and that devices with alleged flow rates of 15-1,500 ml/min should be selected when there is a risk of massive fluid and blood loss. Secondly, varying lengths of outflow tubing also affects the fluid temperature at the point of entering the patient in a length-dependent manner [
10,
12]. To control these contributing factors, we used extension lines and 3-way connectors to achieve equal lengths of tubing extending from each device to the outlet point (108 cm), extending from the outlet point to the distal point (90 cm), and finally extending from the distal point to the patient's intravenous cannula (30 cm). The infusion rate was then set at 400 ml/h as not only does intravenous fluid administration at a rate of 4 ml/kg/h reduces postoperative mortality, but we do routinely employ restrictive fluid management in cases without the risk of massive blood loss [
15]. Under these conditions, the present study further showed that the delivery temperature of 37℃ at the outlet point (108 cm from the warming device) can be achieved using the MAK (set to 38℃) but is not achievable using the Standard Ranger (set to 41℃). Therefore, the MAK may be suitable for preventing intraoperative hypothermia during minor surgery with no anticipated massive bleeding or fluid loss. However, it should be noted that the delivery temperature of 37℃ at the distal point (198 cm from the warming device) was not achieved using either device. Furthermore, the temperature of the delivered intravenous fluid decreased from 34.7 to 32.8℃ using the Standard Ranger and from 37.4 to 35.4℃ using the MAK over a distance of 90 cm from the outlet point.
Although the use of the MAK facilitated achieving an outlet temperature of 37.4℃ at 400 ml/h, the distal temperature of 35.4℃ was insufficient to maintain normothermia during the intraoperative period at a room temperature of approximately 23℃. As a result, Teso tended to decrease across all groups, with impaired central thermoregulation (which controls body heat redistribution) the likely cause during anesthesia [
16]. The expected change in MBT calculated using Horowitz's formula [
12] (previously mentioned in the materials and methods section) was -0.02 ± 0.01℃ in group M, -0.09 ± 0.02℃ in group R, and -0.32 ± 0.05℃ in group C. In addition, Kim et al. [
17] recently reported that the core body temperature at the end of surgery decreased by 0.5 ± 0.5℃ from baseline values in a study using the MAK. In contrast, our study found that use of the MAK resulted in a 0.7℃ temperature increase when compared with the Standard Ranger and the need for intervention to treat hypothermia was less frequent. The observed change in the Teso was -0.6 ± 0.3℃ in group M, -1.2 ± 0.2℃ in group R, and -1.5 ± 0.4℃ in group C. These discrepancies between the expected and observed changes could be attributed to the patient's inability to maintain their core temperature, as well as to heat loss through exposed body surfaces during anesthesia and spinal surgery. Unfortunately, use of the MAK and Standard Ranger devices did not prevent redistribution hypothermia and the Teso decreased by approximately 0.8 and 1.4℃ in groups M and R, respectively. Accordingly, it is not surprising that warming the infusion fluid using the Standard Ranger resulted in a higher frequency of use of the forced-air convective warming device.
This study had several limitations. First, we used a fixed flow rate, while flow rates do vary in clinical practice. Hence, the range of flow rates at which the MAK is capable of warming intravenous fluid to 37℃ remains to be determined. Second, the risk of air embolus, which may form as the MAK does not include a filter assembly, was not evaluated. However, the risk of formation of air emboli is increased in devices that rapidly deliver warmed fluid using a pressurized intravenous infusion method, with one fatality from an air embolus reported to date [
18]. In this regard, the MAK is not designed to infuse intravenous fluid rapidly. Finally, we cannot provide evidence of the safety and efficacy of warming blood products as there is no clinical or laboratory data available. Therefore, until the relevant data becomes available, we recommend that the MAK should be used for maintaining fluid balance in cases where high flow rates are not required. We further recommend the use of an additional air filter to prevent air embolus formation.
In conclusion, our study showed that the Mega Acer Kit, a new HHBC containing a fluid warming device, maintained the distal esophageal temperature more effectively and decreased the incidence of a forced-air convective warming device application more significantly than the Standard Ranger, at a flow rate of 400 ml/h in patients undergoing spinal surgery.
Fig. 1
Clinical and experimental set up. (A) Group R received intravenous fluids using the Standard Ranger (Arizant Healthcare Inc., MN, USA). (B) Group M received intravenous fluids using the Mega Acer Kit® (Ace Medical, Seoul, Korea). Temperature monitoring sites were set up at 3 points; 1) the inlet point (T1), 2) the outlet point (T2, 108 cm from the warming devices), and 3) the distal point of the warmer (T3, 198 cm from the warming device). IP: infusion pump, MAK: the Mega Acer Kit®, R: the Standard Ranger, V: ventilator.
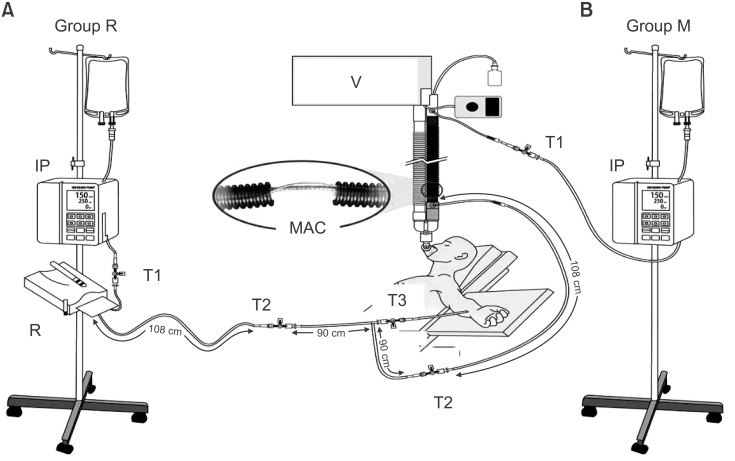
Fig. 2
Changes in distal esophageal temperature (Teso). Teso tended to decrease across all 3 groups. Teso in group M was significantly higher when compared with groups C and R throughout the study period. Group C received intravenous fluids without warming and Group R received intravenous fluids using the Standard Ranger. Group M received intravenous fluids using the Mega Acer Kit®. *P < 0.05 compared with group C, †P < 0.05 compared with group R.

Fig. 3
Changes in fluid temperatures (TFs). TF2 (panel A) and TF3 (panel B) were significantly higher in group M at all time points when compared with Groups C and R. Values in group R were significantly higher when compared with group C. Group C received intravenous fluids without warming and Group R received intravenous fluids using the Standard Ranger. Group M received intravenous fluids using the Mega Acer Kit®. TF1, fluid temperature at the inlet point (T1) of the warming devices; TF2, fluid temperature at the outlet point (T2) 108 cm from the warming devices; TF3, fluid temperature at the distal point (T3) 198 cm from the warming devices. *P < 0.05 compared with the group C, †P < 0.05 compared with the group R.
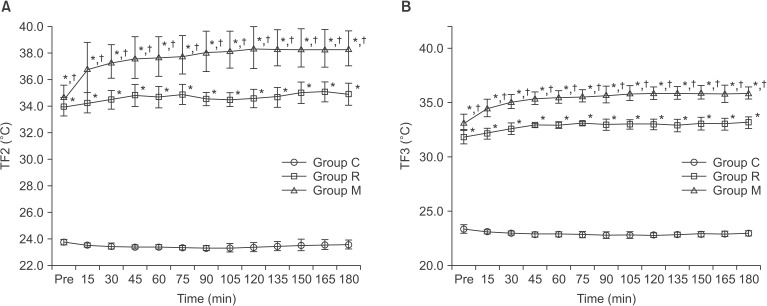
Table 1
Demographic and Intraoperative Data
|
Group C
(n = 30) |
Group R
(n = 30) |
Group M
(n = 30) |
|
Gender (M/F) |
12/18 |
16/14 |
16/14 |
|
ASA (I/II) |
23/7 |
18/12 |
19/11 |
|
Age (yr) |
49.1 ± 10.4 |
47.2 ± 10.6 |
49.3 ± 12.1 |
|
Height (cm) |
162.3 ± 10.3 |
164.6 ± 7.7 |
164.3 ± 8.3 |
|
Weight (kg) |
63.6 ± 10.0 |
62.5 ± 11.1 |
65.9 ± 10.3 |
|
BMI |
24.1 ± 2.1 |
22.9 ± 2.7 |
24.3± 2.4 |
|
EBL (ml) |
572.5 ± 179.6 |
590.8 ± 132.0 |
609.9 ± 133.0 |
|
Urine output (ml) |
412.7 ± 77.3 |
436.7 ± 71.7 |
455.7 ± 110.7 |
Table 2
Intraoperative Esophageal Temperatures and the Estimated Change in Mean Body Temperature (℃)
|
Group C
(n = 30) |
Group R
(n = 30) |
Group M
(n = 30) |
|
Teso, baseline |
36.3 ± 0.2 |
36.4 ± 0.2 |
36.4 ± 0.2 |
|
Teso, maximal declined |
34.6 ± 0.3 |
34.9 ± 0.2*
|
35.6 ± 0.2*,†
|
|
Teso, final |
34.8 ± 0.3 |
35.1 ± 0.1*
|
35.8 ± 0.3*,†
|
|
Δ Teso (max) |
-1.7 ± 0.4 |
-1.4 ± 0.3*
|
-0.8 ± 0.2*,†
|
|
Δ Teso (final) |
-1.5 ± 0.4 |
-1.2 ± 0.2*
|
-0.6 ± 0.3*,†
|
|
Δ MBT (estimated, final) |
-0.32 ± 0.05 |
-0.09 ± 0.02*
|
-0.02 ± 0.01*,†
|
|
Intervention for hypothermia (n) |
30 |
17*
|
0*,†
|
Table 3
The Temperature (℃) of Infusing Fluid at the Inlet, Outlet, and Distal Points from Each Warming Device
|
Group C
(n = 30) |
Group R
(n = 30) |
Group M
(n = 30) |
|
TF1 (Inlet) |
23.8 ± 0.1 |
24.0 ± 0.7 |
24.0 ± 0.8 |
|
TF2 (Outlet) |
23.5 ± 0.3 |
34.7 ± 0.8*
|
37.4 ± 1.7*,†
|
|
TF3 (Distal) |
23.0 ± 0.3 |
32.8 ± 0.6*
|
35.4 ± 1.0*,†
|













