|
 |
|
|
Abstract
Background
Dexmedetomidine is an ╬▒2-adrenoreceptor agonist with sedative, analgesic and anxiolytic effects, and it has more selective ╬▒2-adrenergic effect than clonidine. We evaluate the effect of preansethetic dexmedetomidine 1 ┬Ąg/kg single infusion on sedation, hemodynamics, anesthetic consumption, and recovery profiles during anesthesia.
Methods
Forty-two female patients with American Society of Anesthesiologists physical status I or II undergoing gynecologic surgery with anticipated operation time of 2 h, were randomly assigned to receive dexmedetomidine 1 ┬Ąg/kg (Dex group) or saline (control group) iv over 10 min before anesthetic induction. After tracheal intubation with propofol 2 mg/kg, cisatracurium 0.15 mg/kg iv, anesthesia was maintained with sevoflurane, O2 50%, N2O 50% around a BIS value of 40.
Results
After study drug infusion, BIS of Dex group was lower than that of control group (93.9 ┬▒ 3.1 vs 51.5 ┬▒ 5.2, P < 0.05). Mean arterial pressure (MAP) and heart rate (HR) after intubation were increased in control group, but did not change in Dex group. During maintenance, there was no difference in MAP between groups, but HR of Dex group was lower compared to that of control group. End-tidal concentration (2.0 ┬▒ 0.5 vol% vs 1.4 ┬▒ 0.3 vol%, P < 0.05) and total cumulative consumption of sevoflurane (34.6 ┬▒ 3.8 ml vs 26.5 ┬▒ 5.3 ml, P < 0.05) were lower in Dex group than in control group. Recovery profiles, modified Aldrete score, postoperative nausea vomiting, and visual analogue pain score were not significantly different between groups.
Dexmedetomidine is an ╬▒2-adrenoreceptor agonist and close relative of clonidine that utilizes receptors distinct from the ╬│-aminobutyric acid receptor for benzodiazepines and propofol [1,2]. It is a relatively selective ╬▒2 receptor agonist with sedative and analgesic effects without respiratory depression [2]. Dexmedetomidine has been used for anesthetic practices such as sedation during mechanical ventilation, procedural sedation, postoperative anxiolysis, the prevention of emergence agitation, and treatment of substance withdrawal [3-5]. The several studies have reported the benefits of using dexmedetomidine including neuroprotection, cardioprotection, and renoprotection [6].
While a good anesthetic adjuvant, dexmedetomidine is not routinely used in general anesthetic practice because of the risk of hypotension or bradycardia [7]. Previous studies on the efficacy and safety of dexmedetomidine for general anesthesia have recommended dexmedetomidine 0.5-1 ┬Ąg/kg loading with 0.5-1.0 ┬Ąg/kg/min infusion during intravenous or volatile anesthesia [8,9]. In our present study, we used preanesthetic dexmedetomodine 1 ┬Ąg/kg loading without continuous infusion as a simple anesthetic adjuvant for gynecologic surgeries with an operation time of 2 h. For the accurate measurement of volatile anesthetic consumption, we used the multifunctional anesthetic system (Zeus┬«, Dr├żger, L├╝beck, Germany) for anesthesia.
Thus, the aim of this study was to investigate the effects of preanesthetic dexmedetomdine 1 ┬Ąg/kg on sedation, hemodynamics, anesthetic consumption, and recovery profiles during gynecologic surgeries with an anesthesia time of 2 h.
The Institutional Review Board approved this study and all patients provided informed consent. Forty-two female patients (age range 25-55 years) scheduled for gynecologic surgery with an anticipated operation time of 2 h and classified as American Society of Anesthesiologists physical status I or II were enrolled in the study. Patients with a history of hypertension, hypotension, diabetes mellitus, dysrhythmia, cerebrovascular disease, or previous anesthetic exposure within 1 year were excluded. This study was controlled, double-blinded, and randomized using a sealed envelope technique that placed patients into one of two groups. The envelope was opened by an independent anesthetist who prepared the study drugs but was not otherwise involved in the study.
All patients were premedicated with glycopyrrolate 0.2 mg IM 1 h before anesthesia. The standard monitor used in preoperative area included an electrocardiogram, non-invasive blood pressure, a pulse oximeter, end-tidal (Et) gas measurement, and a train-of-four (TOF) monitor. We used a multifunctional anesthetic machine (Zeus┬«, Dr├żger, L├╝beck, Germany) that could measure the consumption of volatile anesthetics. Before the start of study, O2 was flushed 3 times per minute for 5 minutes to ensure denitrogenation.
Patients were blinded and randomized to receive a 10 minute infusion of either normal saline 10 ml (control group) or dexmedetomidine (Precedex┬«, Hospira, Lake Forest, USA) 1 ┬Ąg/kg in normal saline 10 ml (dexmedetomidine group, Dex group) before anesthetic induction, After preoxygenation with O2 at 8 L/min, all patients received propofol 2 mg/kg and cisatracurium 0.15 mg/kg intravenously (iv) for tracheal intubation. Anesthesia was maintained with sevoflurane with O2 (1.5 L/min) and N2O (1.5 L/min). Anesthetic depth was monitored using the Bispectral Index (BISŌäó, A-2000 monitor, Aspect medical system, Newton, USA) to reach a target value of around 40 by manipulating sevoflurane vaporizer setting. Ventilation was controlled to maintain an Et CO2 of around 30-35 mmHg. For hemodynamic stability, fentanyl 1 ┬Ąg/kg iv was administered if the patient's mean arterial pressure (MAP) was more than 20% above the baseline value (value before anesthetic induction) while decreases in MAP of a similar magnitude were treated with ephedrine 4-8 mg iv and also glycopyrrolate 0.1 mg iv if the patient's heart rate (HR) was less than 45 beats/min. Neuromuscular block was monitored during the operation and incremental doses of 0.05 mg/kg cisatracurium were given when a TOF stimulus produced two twitches. Residual muscle paralysis was antagonized using glycopyrrolate and pyridostigmine iv. After skin closure, all anesthetics (sevoflurane, N2O) were turned off, and the ventilation was controlled with O2 (6 L/min) until extubation. The endotracheal tube was extubated when adequate spontaneous ventilation (tidal volume > 4 ml/kg), a BIS of greater than 90, and patient responses to verbal commands were established. In the recovery room, all patients received humidified O2 via facemask while SpO2 and HR were monitored.
BIS, MAP, and HR were recorded at several time points as follows: before induction (baseline), after the end of study drug infusion, before intubation, immediately after intubation, 10, 20, 30, 60, and 90 minutes after intubation, and in the recovery room. SpO2 was measured before induction (baseline) and after the end of study drug infusion. Et sevoflurane concentration and the cumulative doses of sevoflurane consumed were measured at 10, 20, 30, 60, and 90 minutes after intubation.
To assess the recovery profiles, we measured the times taken to reach sevoflurane Et 0.8%, to respond to a suction catheter, to obey verbal commands, and to complete tracheal extubation after turning off the vaporizer. After the patient's arrival in the recovery room, we observed the time taken to reach a modified Aldrete score of 9 [10], noted the existence of postoperative nausea vomiting (PONV), and quantified postoperative pain by visual analogue score (VAS; 0 = no pain; 10 = worst possible pain). The above parameters were measured and recorded by the observer who was blinded to the study group assignment.
According to a power analysis, a sample size of 21 patients per study group was determined to be sufficient for identifying a 20% difference between the two groups' hemodynamic changes with a power of 0.8 and ╬▒ value of 0.05 with the reference to Basar et al. [8]. All data are expressed as means ┬▒ SD or numbers. Statistical analysis was performed using SPSS Inc (version 14, Chicago, USA).
Ages, height, weight, duration of surgery, and hemodynamics between groups, were analyzed using an unpaired t test. Types of operation, rescue drug, PONV were analyzed using Chi-square (type of operation) or Fisher's exact test (rescue drug, PONV). Recovery profiles except PONV, were analyzed using an unpaired t test. The hemodynamic variables within each group were analyzed by repeated measures of analysis of variance (ANOVA), and post-hoc comparisons were performed using Dunnett's test. A P value of less than 0.05 was considered to be statistically significant.
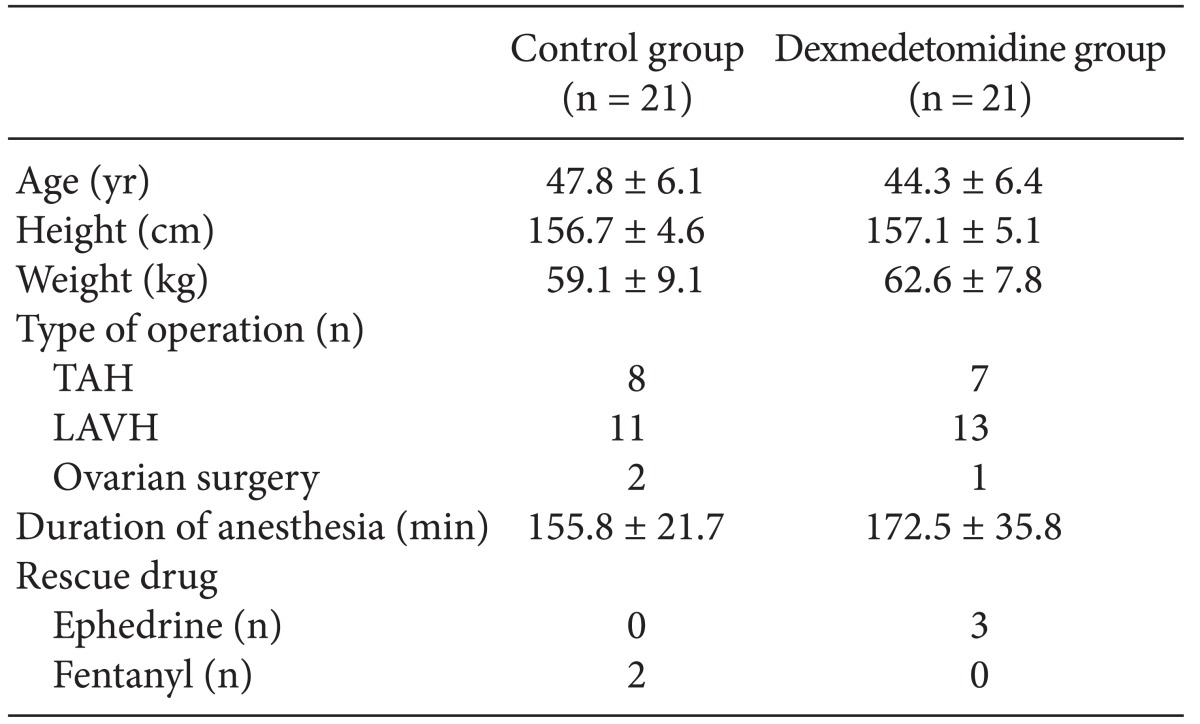
Forty-two patients were enrolled in the study and randomized into groups. No patients were excluded in analysis. Two groups did not differ significantly in age, height, weight, or duration of anesthesia (P > 0.05, Table 1).
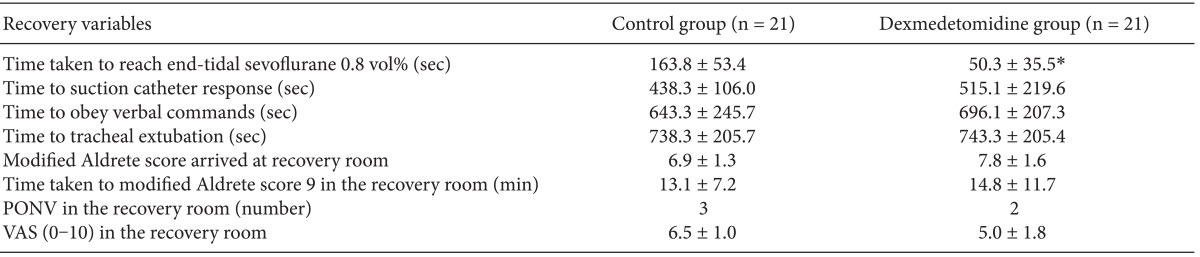
After infusion of the study drug was completed, BIS of Dex group was significantly lower than that of control group (51.5 ┬▒ 5.2 vs 93.9 ┬▒ 3.1, P = 0.000) (Fig. 1) without respiratory depression (for SpO2 99.5 ┬▒ 0.8% vs 98.0 ┬▒ 1.4%, P > 0.05: control vs Dex group).
After tracheal intubation, both MAP and HR significantly increased in control group (P = 0.010 for MAP, P = 0.022 for HR) but remained unchanged in Dex group. During maintenance, there were no significant differences in MAP between groups, but HR of Dex group was significantly lower compared with that of control group (P = 0.034) (Fig. 2). For hemodynamic stability, fentanyl was injected in 3 patients in control group and ephedrine was injected in 2 patients in Dex group, and glycopyrrolate was not injected in all other patients.
End-tidal concentration (at 90 min; 2.0 ┬▒ 0.5 vol% vs 1.4 ┬▒ 0.3 vol%, P = 0.029, P < 0.05: control vs Dex group) and total cumulative consumption dose of sevoflurane (at 90 min; 34.6 ┬▒ 3.8 ml vs 26.5 ┬▒ 5.3 ml, P = 0.017, control vs Dex group) were significantly lower in Dex group compared with control group at 20 min, 30 min, 60 min, and 90 min after intubation (Fig. 3). The time taken to reach sevoflurane Et 0.8 vol% was significantly shorter in Dex group compared with control group (P = 0.008, Table 2). However, as shown by recovery profiles, the time taken to respond to a suction catheter, to obey verbal commands, and to complete tracheal extubation after turning off the vaporizer were similar between groups (P > 0.05, Table 2). In addition, the time taken to reach a modified Aldrete score of 9, PONV, and VAS score in the recovery room were not different between two groups (P > 0.05, Table 2).
This study shows that preanesthetic dexmedetomidine 1 ┬Ąg/kg single infusion prevents hemodynamic changes by tracheal intubation and reduces the total cumulative consumption of sevoflurane. However, Dex group and control group had similar recovery profiles, modified Aldrete scores, PONV and VAS scores.
Dexmedetomidine is a highly selective ╬▒2 agonist that results in sedation and anxiolysis. It binds to transmembrane G protein-binding adrenoreceptors in the brain and spinal cord with a dose-dependent ╬▒2-selectivity that is approximately 7- to 8-fold greater than that of clonidine [11]. Stimulation of ╬▒2-adrenoreceptor subtypes mediates sedative and antinociceptive actions (╬▒2A) and a vasoconstrictive cardiovascular effect (╬▒2B), and modulates dopaminergic neurotransmission, hypothermia and a variety of behavioral responses (╬▒2C) [12]. The inhibition of norepinephrine release suppresses the level of excitation, especially in the locus coeruleus (╬▒2A) which controls anxiety, arousal, sleep, and opioid withdrawal [12].
When compared to other sedatives, dexmedetomidine has a favorable profile due its ability to produce good sedative outcomes without respiratory depression, an important consideration in terms of emergence [13]. Dexmedetomidine has not been routinely used in elective general anesthesia, especially day surgery, due to intraoperative hemodynamic instability such as hypotension, bradycardia, and delayed emergence [6,7]. On the other hand, dexmedetomidine has been increasingly popularity as an adjuvant to general anesthesia since it features advantages such as anesthetic sparing, hemodynamic stability, and the reduction of emergence agitation [14-16].
Aantaa [17] reported that dexmedetomidine 0.5-1.0 ┬Ąg/kg induced sedation within 5 minutes and reached its maximum effects at 15 minutes and we had the same results. Generally sedation induced by dexmedetomidine was similar to arousable sedation like normal sleep [17].
Tracheal intubation and extubation evoke strong hemodynamic stress responses but most patients tolerate the stress well without serious sequale. However, it can lead to myocardial ischemia in patients with pre-existing severe coronary artery disease. In these patients, the use of dexmedetomidine may lower the risk of coronary ischemic events by lowering the rate-pressure product [18]. Additionally, dexmedetomidine has been used to successfully facilitate the withdrawal of ventilation in ICU patients who previously failed weaning attempts because of agitation [18]. Adverse reactions to dexmedetomidine include hypotension, bradycardia and even sinus arrest in healthy young volunteers with high vagal tone secondary to the attenuation of plasma catecholamine release [19]. In particular, large doses or rapid injection of dexmedetomidine have been associated with these adverse events [1]. Dexmedetomidine (over 1.0 ┬Ąg/kg) should be infused over 10 minutes and titrated to an adequate dosage on a case by case basis. We achieved stability in MAP by slowly infusing dexmedetomidine over 10 min at an adequate dosage for the patients. The over-infusion or over-dosage of anesthetics should be prevented by BIS monitoring. We maintained equal anesthetic depth in both groups at a BIS value of about 40 by controlling sevoflurane vaporizer setting. Intraoperative co-administration of dexmedetomidine with other anesthetics could potentiate any hemodynamic instability by over-sedation or over-dosing. Therefore, use of an anesthetic depth monitor like BIS is essential when employing a dexmedetomidine adjuvant for general anesthesia. During intravenous or volatile anesthesia, dexmedetomidine is a less appropriate adjuvant for propofol or remifentanil anesthesia when compared to volatile anesthetics because of the centrally mediated vagotonic or sympatholytic actions of propofol and remifentanil [20,21].
Dexmedetomidine shows the biphasic hemodynamic effect that not only vasodilation by presynaptic effect on sympathetic and postsynaptic effect on central nervous system at high concentration but also vasoconstriction by postsynaptic effect on vascular system at low concentration (an initial increase in MAP followed by reduction in MAP and HR) [22]. We did not observe the biphasic effects of dexmedetomidine in this study, perhaps because the slow infusion of dexmedetomidine over 10 minutes may have caused low plasma concentrations and reduced MAP during induction period.
Our results showed that preanesthetic dexmedetomidine 1 ┬Ąg/kg reduces the Et sevoflurane concentration by 30% (2.0 vol% vs 1.4 vol%) and total consumption of sevoflurane by 23.4% (34.6 ml vs 26.5 ml) compared with control group. In previous studies of anesthetic consumption, dexmedetomidine has been shown to reduce target propofol concentration by 30-50% during propofol-remifentanil anesthesia [9] and end-tidal concentration by 15-20% during volatile anesthesia [23]. The pharmacoeconomic effect of dexmedetomidine may aid in reducing the concentration of anesthetics used and preventing adverse effects such as hepatic and renal toxicity, severe myocardial depression, and the greenhouse effect.
Although the time to reach Et sevoflurane 0.8 vol% was faster in Dex group compared with control group during emergence, all patients had similar recovery profiles. A possible explanation is that the analgesic and sedative effects of dexmedetomidine may be in effect during the perioperative period, making it possible for patients in Dex group to reach the same BIS value at a lower Et sevoflurane concentration. Isik et al. [24] reported that in children undergoing magnetic resonance imaging, dexmedetomidine 1.0 ┬Ąg/kg iv after anesthetic induction was effective in the reduction of agitation, but prolonged the removal of LMA and eye opening time. These findings might be attributed to the sustained therapeutic plasma concentrations of dexmedetomidine relative to the short procedure time (45-49 min).
The elimination half-life offer dexmedetomidine is 2-3 h with a context-sensitive half-time ranging from 4 minutes after a 10 minute infusion to 250 minutes after an 8 hr infusion [25]. Iirola et al. [26] recently demonstrated that the pharmacokinetic of dexmedetomidine is affected by low cardiac output, increasing age, and low plasma albumin; for example, elimination half-life and context-sensitive half-time are prolonged in the elderly. They reported that context-sensitive half times of a 40 yr-old patient were 20, 50, and 55 min after a 1, 2, and 3 h infusion, respectively, with no further increases in the context-sensitive half time beyond an infusion period of 3 h. The duration of operation, drug infusion time, and context-sensitive half times must be considered when planning anesthesia for patients. In the present study, 2 h operation time of each gynecologic surgery was similar to the elimination half-life of dexmedetomidine; so it is possible for proper recovery to occur without sedation in Dex group, resulting in similar recovery profiles between the two groups [13]. It is well known that dexmedetomidine significantly lower rates of postoperative delirium when compared to midazolam or propofol [7]. Our results suggest that preansethetic dexmedetomidine 1 ┬Ąg/kg may be a good adjunct for anesthesia for an anticipated operation time of 2-hr.
Some studies have reported that the postoperative analgesia of dexmedetomidine is evident in the recovery room [27,28], but did not continue after recovery room discharge [28]. This may be related to the elimination half-life of dexmedetomidine [13]. With increasing doses of dexmedetomidine for analgesia, there should be higher incidences of hypotension and delayed emergence [29]. In McQueen-Shadfar et al. [29] retrospective investigation of dexmedetomidine 0.2-0.7 ┬Ąg/kg/h infusion during gynecologic operations, there was no difference in pain score, analgesics, or rescue antiemetic between Dex group and control group, but Dex group stayed for longer in the recovery room. Using a single pre-induction infusion of dexmedetomidine 2 ┬Ąg/kg, Lawrence and De Lange [30] reported reduced analgesic use, antiemetic and a higher occurrence of hypotension and bradycardia despite similar findings of perioperative hemodynamic stability and lower isoflurane concentration. In the present study, we only selected patients having gynecological surgery in the lower abdominal region; but if the type of surgery were more invasive or of a longer operating time, it may be necessary to increase the loading dose or the duration of continuous infusion of dexmedetomidine.
In conclusion, preanesthetic dexmedetomidine 1 ┬Ąg/kg single infusion is a good anesthetic adjuvant method for general anesthesia that can attenuate the hemodynamic response to tracheal intubation and have the advantage of anesthetic consumption saving without the change of recovery profiles.
References
1. Nishikawa K, Kubo K, Obata H, Yanagawa Y, Saito S. The influence of manipulations to alter ambient GABA concentrations on the hypnotic and immobilizing actions produced by sevoflurane, propofol, and midazolam. Neuropharmacology 2011; 61: 172-180. PMID: 21497611.


3. Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care 2000; 4: 302-308. PMID: 11056756.



4. Patel A, Davidson M, Tran MC, Quraishi H, Schoenberg C, Sant M, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg 2010; 111: 1004-1010. PMID: 20705788.


5. Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 2008; 21: 457-461. PMID: 18660652.


6. Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol 2008; 4: 619-627. PMID: 18484919.


7. Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 2011; 71: 1481-1501. PMID: 21812509.


8. Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O. The effects of preanesthetic, single-dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. J Clin Anesth 2008; 20: 431-436. PMID: 18929283.


9. Kang WS, Kim SY, Son JH, Kim JD, Muhammad HB, Kim SH, et al. The effect of dexmedetomidine on the adjuvant propofol requirement and intraoperative hemodynamics during remifentanil-based anesthesia. Korean J Anesthesiol 2012; 62: 113-118. PMID: 22379564.



10. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995; 7: 89-91. PMID: 7772368.


11. Murthy TVSP, Singh R. Alpha 2 adrenoreceptor agonist-dexmedetomidine role in anaesthesia and intensive care: a clinical review. J Anaesth Clin Pharmacol 2009; 25: 267-272.
12. Arcangeli A, D'Al├▓ C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets 2009; 10: 687-695. PMID: 19702517.


13. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000; 93: 382-394. PMID: 10910487.


14. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg 2008; 106: 1741-1748. PMID: 18499604.


15. Wijeysundera DN, Naik JS, Beattie WS. Alpha-2 adrenergic agonists to prevent perioperative cardiovascular complications: a meta-analysis. Am J Med 2003; 114: 742-752. PMID: 12829201.


16. But AK, Ozgul U, Erdil F, Gulhas N, Toprak HI, Durmus M, et al. The effects of pre-operative dexmedetomidine infusion on hemodynamics in patients with pulmonary hypertension undergoing mitral valve replacement surgery. Acta Anaesthesiol Scand 2006; 50: 1207-1212. PMID: 16978159.


17. Aantaa R. Assessment of the sedative effects of dexmedetomidine, an alpha 2-adrenorecptor agonist with analysis of saccadic eye movements. Pharmacol Toxicol 1991; 68: 394-398. PMID: 1682907.


18. Kulkarni A, Price G, Saxena M, Skowronski G. Difficult extubation: calming the sympathetic strom. Anaesth Intensive Care 2004; 32: 413-416. PMID: 15264740.


19. Ingersoll-Weng E, Manecke GR Jr, Thistlethwaite PA. Dexmedetomdine and cardiac arrest. Anesthesiology 2004; 100: 738-739. PMID: 15108994.


20. Elliott P, OŌĆÖHare R, Bill KM, Phillips AS, Gibson FM, Mirakhur RK. Severe cardiovascular depression with remifentanil. Anesth Analg 2000; 91: 58-61. PMID: 10866887.


21. Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedatives, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg 2000; 90: 699-705. PMID: 10702460.


22. Snapir A, Posti J, Kentala E, Koskenvuo I, Sundell I, Tuunanen H, et al. Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology 2006; 105: 902-910. PMID: 17065883.


23. Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effect of dexmedetodmine on isoflurane requirements in healthy volunteers. I: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth 1999; 83: 372-380. PMID: 10655905.



24. Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth 2006; 16: 748-753. PMID: 16879517.


25. Venn RM, Karol MD, Grounds RM. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Br J Anaesth 2002; 88: 669-675. PMID: 12067004.



26. Iirola T, Ihmsen H, Laitio R, Kentala E, Aantaa R, Kurvinen JP, et al. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth 2012; 108: 460-468. PMID: 22277665.



27. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomdine reduces perioperative analgesic requirements. Can J Anaesth 2006; 53: 646-652. PMID: 16803911.


28. Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl of dexmedetomidine combined with desflurane for bariatric surgery. J Clin Anesth 2006; 18: 24-28. PMID: 16517328.


29. McQueen-Shadfar LA, Megalla SA, White WD, Olufolabi AJ, Jones CA, Habib AS. Impact of intraoperative dexmedetomidine on postoperative analgesia following gynecologic surgery. Curr Med Res Opin 2011; 27: 2091-2097. PMID: 21916531.


30. Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia 1997; 52: 736-744. PMID: 9291757.


Fig.┬Ā1
Changes in the Bispectral index (BIS) during sevoflruane anesthesia. *P < 0.05 compared with control group, ŌĆĀP < 0.05 compared with the value before induction, ŌĆĪP < 0.05 compared with the value at the end of study drug infusion.
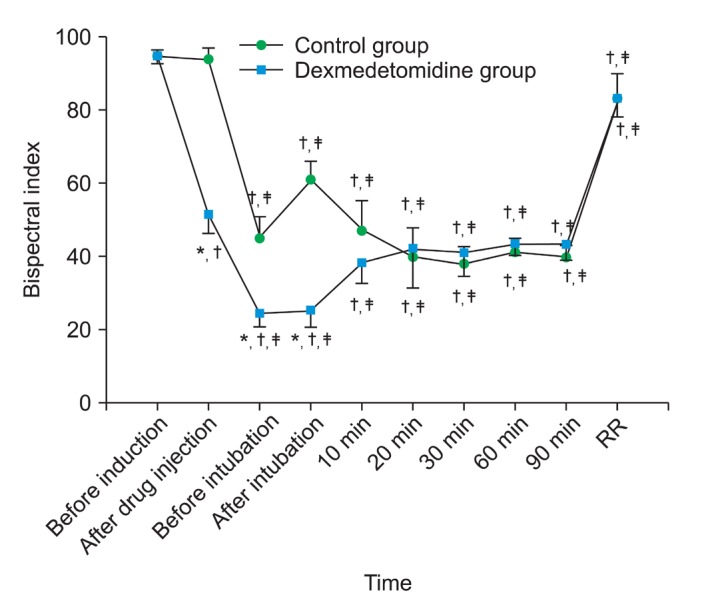
Fig.┬Ā2
Changes in (A) heart rate and (B) mean arterial pressure (MAP) during sevoflurane anesthesia. *P < 0.05 compared with control group, ŌĆĀP < 0.05 compared with the value before induction, ŌĆĪP < 0.05 compared with the value at the end of study drug infusion.
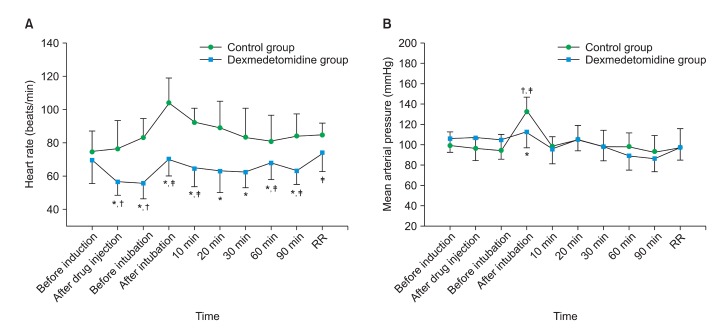
Fig.┬Ā3
Changes in (A) the endtidal concentration of sevoflurane and (B) total cumulative consumption of sevoflurane (mean ┬▒ SD) during sevoflurane anesthesia. *P < 0.05 compared with control group.
Table┬Ā1
Characteristics of Female Patients Scheduled for Gynecological Surgery Who Were Entered into The Study
- TOOLS