Introduction
Cardiopulmonary bypass (CPB) and aortic cross-clamp (ACC) induce various perioperative inflammatory reactions and postoperative organ injury, contributing to postoperative organ dysfunction of heart, lungs, and kidneys after a cardiac surgery as well as increase perioperative morbidity and mortality. Upon exposure to the CPB circuit, circulating humoral and cellular factors activate neutrophils and the complement system to induce systemic inflammatory processes [
1-
3]. Consequently, analyses of serum inflammatory cytokines such as tumor necrosis factor (TNF)-╬▒ and interleukin (IL)-6 have been used to determine the degree of systemic inflammation under various clinical conditions [
3,
4].
Many studies have examined the anti-inflammatory and protective effects of ulinastatin, a urinary trypsin inhibitor, against ischemia-reperfusion organ injury [
5-
7]. Their results indicate that ulinastatin suppresses neutrophil infiltration and reduces the release of elastase and chemical mediators produced by neutrophils [
8-
10]. Other clinical trials have described the inhibitory effects of ulinastatin on CPB-induced proinflammatory cytokine release and cardiopulmonary dysfunction, and stable hemodynamics during the post-CPB period [
11-
13]. However, those studies did not fully evaluate the positive effects of ulinastatin on postoperative organ dysfunction of the heart, lungs, and kidneys in relation to the attenuated release of proinflammatory cytokines during a cardiac surgery.
We hypothesized that ulinastatin pretreatment would attenuate CPB-induced inflammation and major organ injury. Therefore, in this study, we evaluated whether ulinastatin pretreatment would improve postoperative major organ function and attenuate proinflammatory cytokine release during a cardiac valve surgery.
Materials and Methods
After obtaining the approval from our Institutional Review Board, 30 patients undergoing an elective cardiac surgery were enrolled between March 2008 and December 2008. Informed consent was obtained from all subjects prior to the procedure. Preoperative exclusion criteria were: urgent/emergency surgery, previous heart surgery, diabetes, ischemic heart disease, combined surgery with a coronary artery bypass graft procedure, age >75 years, left ventricular ejection fraction <45%, diabetes, active gastropathic disorder, chronic obstructive pulmonary disease, preoperative administration of furosemide, and renal failure requiring renal replacement therapy. The intraoperative exclusion criteria included: intraoperative use of an intra-aortic balloon pump (IABP), intraoperative administration of steroids or tranexamic acid, and intraoperative transfusion of fresh frozen plasma (FFP) or platelet concentrates during CPB. The postoperative exclusion criteria included reoperation for surgical correction of an intractable postoperative bleeding within 2 hours after the end of surgery and transfusion of any banked blood product.
Patient allocation
Twenty-six patients undergoing elective cardiac surgery requiring CPB were assigned to a prospective, double-blinded, randomized fashion to two groups: control (group C, n = 13) and ulinastatin pretreatment (group U, n = 13) during our study period. Patients were allocated to the randomization process using a patient identification number, which was stratified in a 1 : 1 ratio, to receive either ulinastatin (Ulistin; Hallyim Inc., Markham, ON, Canada) or the same volume of normal saline. The investigators and physicians who managed the patients were blinded to the patients' group allocation. All physicians and nursing staff who cared for the patients were unaware of the group allocations. Ulinastatin (5,000 U/kg) or a placebo was administered intravenously 30-40 min before initiating CPB.
Anesthesia and intensive care
Preoperative medication continued until the morning of surgery. Upon patient arrival to an operating room, routine monitoring for cardiovascular surgical patients, including electrocardiogram (ECG), pulse oximetry, bispectral index (BIS; Aspect Medical System Inc., Norwood, MA, USA), and infrared regional cerebral O2 saturation (INVOS; Somanetics, Troy, MI, USA) were started.
After a 20-G catheter was placed in the radial artery for continuous invasive arterial pressure monitoring, anesthesia was induced with a bolus of etomidate (0.1-0.2 mg/kg), a remifentanil infusion of 1.0 ┬Ąg/kg/min, and a target-controlled infusion of propofol (effect-site concentration, 1.0-1.5 ┬Ąg/ml) in all patients. Next, the propofol target concentration was adjusted to maintain a BIS score of 45-60. Tracheal intubation and intraoperative muscle relaxation were facilitated and maintained by a bolus and supplemental administration of rocuronium under the guidance of peripheral nerve stimulator monitoring. After tracheal intubation, patients were mechanically ventilated with volume-controlled ventilation using an oxygen/air mixture (FiO2) of 0.5 at a fixed tidal volume of 8 ml/kg. The respiration rate was adjusted to maintain an end-tidal carbon dioxide tension of 35-40 mmHg.
After inducing anesthesia, a large-bore central venous and pulmonary artery catheter were placed in the internal jugular vein to monitor central venous pressure (CVP), pulmonary arterial pressure (PAP), continuous cardiac output, and mixed venous O2 saturation (SvO2). A transesophageal echocardiography (TEE) probe (Omniplane and Vivid 7; GE Healthcare, Piscataway, NJ, USA) was inserted, and comprehensive TEE was performed. Prior to CPB, the mean arterial blood pressure (MABP) was maintained at 80-120% of the pre-induction value in all patients throughout the entire study period using hydroxyethylstarch (100-250 ml) and phenylephrine (0.01-0.06 ┬Ąg/kg/min).
Ulinastatin (5,000 U/kg) or the same volume of normal saline (placebo) was intravenously administered to group U or C, respectively, over 10 min after the opening of a pericardium.
Heparin (300 IU/kg) was infused to maintain an activated coagulation time of >450 s during CPB. The extracorporeal circuit was primed with 1,500 ml of crystalloid solution consisting of 1,300 ml of balanced solution, 100 ml of 20% mannitol, and 100 ml of 20% albumin. A membrane oxygenator with hollow polypropylene fibers and polyvinyl chloride tubing was used for CPB. The low flow rates during CPB were adjusted to 2.2-2.5 L/min/m2 to maintain a systemic perfusion pressure of 60-70 mmHg. Phenylephrine was administered when necessary to maintain the target systemic pressure. A mild to moderate hypothermic CPB technique (26-28Ōäā) was applied during CPB, and the hematocrit was maintained at 23-25%, but warm modified cardioplegic reperfusion was performed before release of the cross-clamp. Packed red blood cells were transfused in cases of a low hematocrit (<21%). Myocardial protection was achieved by inducing intermittent antegrade and retrograde cold (4Ōäā) crystalloid cardioplegia every 30 minutes and continuous topical cooling during cardiac standstill. PaCO2 was maintained at 40 mmHg at the standard electrode temperature of 37Ōäā (╬▒-stat) during CPB. The patients were weaned from CPB when their rectal temperature reached 36Ōäā. Heparin was neutralized with protamine sulfate.
Intraoperative fluid management was performed upon a routine TEE monitoring of two-dimensional cardiac contour images and Doppler profiles. Dopamine was infused as a first-line inotropic agent to wean the patients from CPB when the rectal temperature was >35Ōäā in all patients. The dopamine infusion rate was adjusted to achieve a cardiac index (CI) >2.0 L/min/m2 and MABP >65 mmHg and continues until full restoration of sufficient hemodynamics without inotropic support.
After the surgery, the patients were taken to the intensive care unit (ICU) and ventilated with institutional routine ventilation with a FiO2 of 0.3-0.5. The patients were weaned off mechanical ventilation as soon as they were awake and breathing faster than the ventilator set rate and extubated when they met the extubation criteria.
Data collection and blood sampling
The following intraoperative variables were determined immediately after anesthesia induction (T1), 1 hour after weaning from CPB (T2), and at the end of surgery (T3): CI, MABP, CVP, mean PAP, heart rate, mixed venous oxygen saturation, serum lactate, hematocrit, arterial O2 tension (PaO2), arterial O2 tension/inspired O2 ratio (PaO2/FiO2 ratio), and BIS. Dopamine infusion less than 5 minutes was excluded from the determination of the dopamine requirement.
Creatine kinase-MB (CK-MB), troponin I (TnI), and serumcreatinine (s-Cr) were measured before the induction of anesthesia (T0) and immediately after the end of anesthesia (T3).
Postoperative blood analysis was performed at 24 hours after the completion of the surgery (POD).
The patients' plasma levels of IL-6 and TNF-╬▒ were determined at T1, T2, and T3. Blood samples were drawn into the tubes containing ethylenediaminetetraacetic acid via the central venous catheter and centrifuged at 3,000 rpm for 20 min. The extracted plasma was stored in polypropylene tubes at -80Ōäā until further analysis and then analyzed for IL-6 and TNF-╬▒ by a quantitative sandwich enzyme immunoassay (Quantikine ELISA; R&D Systems, Inc., Minneapolis, MN, USA). All enzyme immunoassays were performed with a V-MAX 220 VAC ELISA reader (Molecular Devices, Palo Alto, CA, USA).
For primary objectives, PaO2/FiO2, CK-MB, TnI, the glomerular filtration rate (GFR), and creatinine (Cr) clearance were recorded at POD to determine the degree of postoperative major organ dysfunction and their intergroup differences were determined.
The PaO
2/FiO
2 ratio, dopamine requirement, and s-Cr were recorded at T3. The incidence of postoperative myocardial, pulmonary, or renal injury was defined as: elevated TnI (>0.68 ┬Ąg/l) and CK-MB (>40.4 ┬Ąg/l) with a newly developed pathological Q wave on ECG (13); PaO
2/FiO
2 ratio <3.0 with bilateral pulmonary infiltration on the chest radiography without clinical evidence of elevated left atrial pressure; and an increase in s-Cr of >0.3 mg/dl or 50% from baseline within 48 hours as defined by the Acute Kidney Injury Network (AKIN) [
14] or urine output <0.5 ml/kg/h for >6 hrs despite of fluid resuscitation within 24 hours after surgery.
Data analysis
Statistical analyses were performed with SPSS software (Chicago, IL, USA). All values are reported as the mean (standard deviation and 95% confidence interval [CI]) for normally distributed variables or median (25-75th percentile) if the distribution was not normal. Unpaired Student's t-tests or the Mann-Whitney U-test were used for intergroup comparisons of the mean or median values. The Fisher's exact test was used to analyze intergroup differences in the categorical data, including the incidences. A two-way repeated-measures analysis of variance (ANOVA) was used to compare serially recorded variables. If a difference was found, the Student's t-test (or the Mann-Whitney U-test, as appropriate) was used for an intergroup comparison. Repeated-measures one-way ANOVA or Friedman's test were used followed by Dunnett's or Tukey's tests for multiple pairwise comparisons versus T1 values. Statistical significance was set at P < 0.05.
A minimum sample size of 10 in each group was necessary to produce an intergroup and intragroup difference of mean PaO2/FiO2 ratio > 116.4 (30% of mean PaO2/FiO2 ratio, 388.0) with an expected standard difference of 74.7, a power of 0.80 and an alpha of 0.05, based on our pilot data which were previously corrected from 10 previous cardiac surgery patients as baseline values.
Results
Two patients were excluded from group C and one patient was excluded from group U due to the intraoperative administration of tranexamic acid, IABP insertion, and transfusion of FFP or platelet concentrate during CPB.
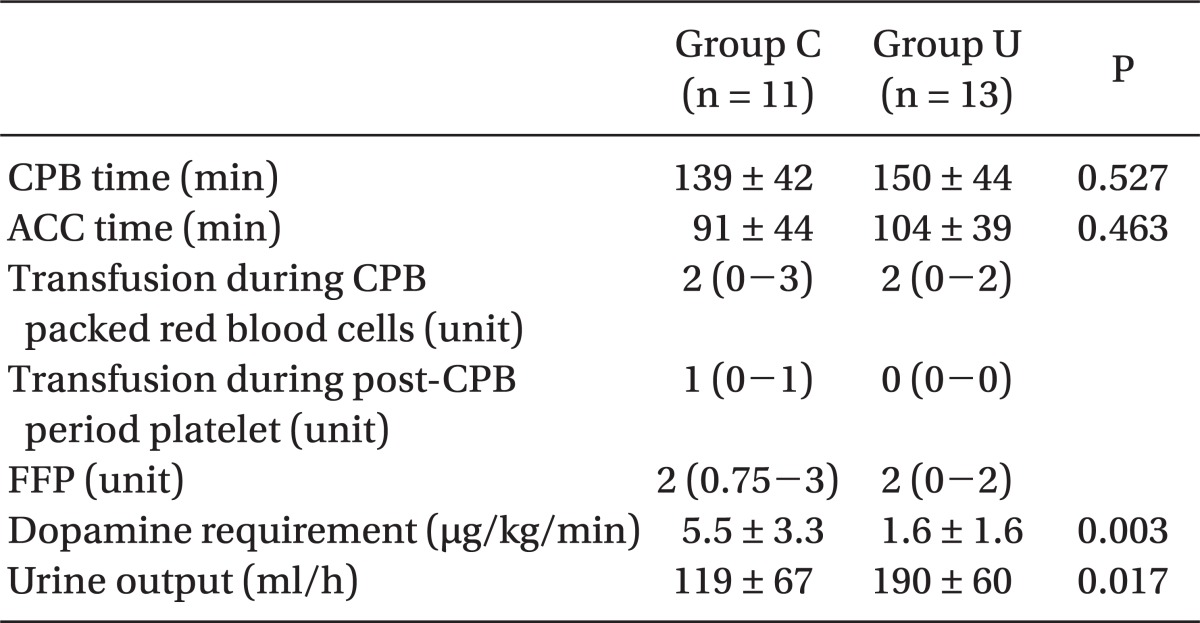
Demographic data and preoperative and intraoperative interventions such as CPB time, ACC time, and amount of banked blood transfused were not different between the groups, except for intraoperative urine output (P = 0.017) (
Table 1 and
2).
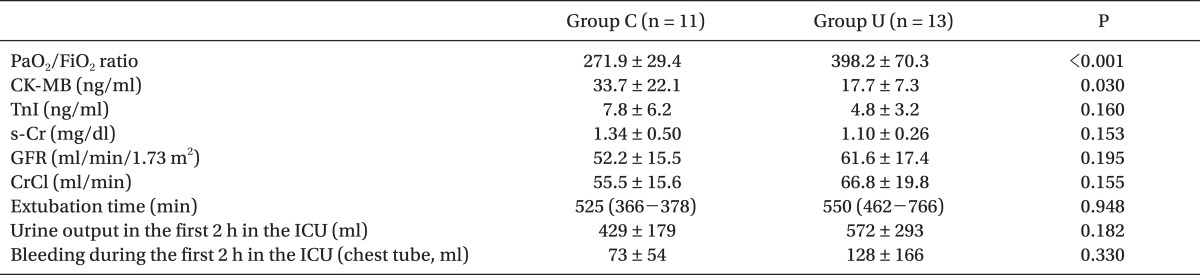
As primary outcomes, the PaO
2/FiO
2 ratio at POD in group U (3.9 ┬▒ 0.6) was significantly higher than that in group C (2.9 ┬▒ 0.6) (P = 0.001). CK-MB at POD in group U was significantly lower than that in group C (P = 0.030), but TnI at POD was not different between the groups. s-Cr, the estimated GFR, and Cr clearance at POD were not different between the groups (
Table 3).
The intraoperative PaO
2/FiO
2 ratio and PaO
2/FiO
2 ratio at T1 and T2 were not different between the groups, but that at T3 in group U (3.8 ┬▒ 0.8, 95% CI, 3.4-4.2) was significantly greater than that in group C (2.8 ┬▒ 0.7; 95% CI, 2.4-3.3, P = 0.005). The dopamine requirement during post-CPB in group U was significantly less than that in group C (P = 0.003) (
Table 4).
The intraoperative levels of IL-6 and TNF-╬▒ at T2 and T3 were significantly greater than those at T1 in both groups, but those at T1, T2, and T3 were not different between the groups (
Table 5).
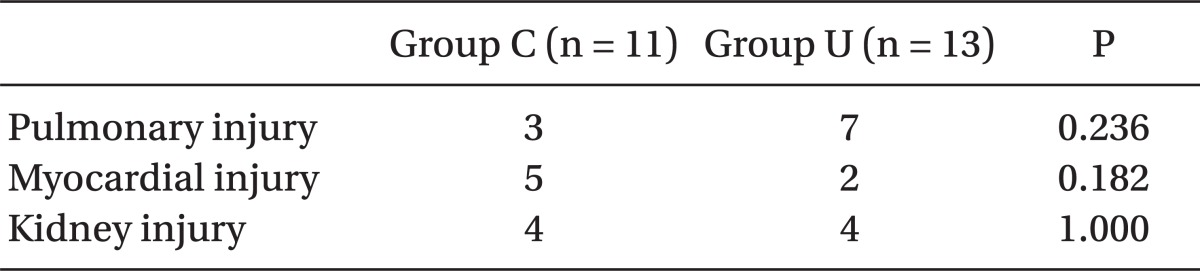
Postoperative urine output was not different between the groups. The incidence of pulmonary injury, myocardial injury, and AKIN was also not different between the groups (
Table 6).
Discussion
This study showed that ulinastatin pretreatment, even at a low dosage (5,000 IU/kg), resulted in better pulmonary function with a significantly higher postoperative PaO
2/FiO
2 ratio and less myocardial injury with a significantly less elevated postoperative CK-MB level. Furthermore, ulinastatin provided better cardiac performance requiring less inotropic support and a higher PaO
2/FiO
2 ratio after weaning from CPB. Our results suggest that a small pretreatment dose of ulinastatin provides more favorable postoperative cardiopulmonary function after applying CPB, in accordance with the previous clinical studies showing favorable clinical profiles. Ulinastatin does not suppress norepinephrine release or post-CPB myocardial enzyme release [
14]; however, it provides a more favorable postoperative lung function [
15]. Ulinastatin administration before CPB improves cardiovascular stability and pulmonary dysfunction, whereas it does not suppress the CPB-induced release of neutrophils or increase myocardial enzyme levels [
11]. The increased PaO
2/FiO
2 ratio following ulinastatin pretreatment suggests a reduction in postoperative morbidity and mortality as shown in other studies [
16-
18].
Ulinastatin pretreatment did not affect other outcomes, including intraoperative and postoperative renal function or the amount of postoperative bleeding thorough a chest tube in this study. The absence of an intergroup difference in the postoperative TnI level, a more specific indicator of myocardial injury, may contradict the efficacy of ulinastatin for attenuating myocardial injury and providing a favorable cardiac function, which were indicated by the reduced release of CK-MB and reduced the need for inotropic support in our study.
However, we did not find any attenuation of proinflammatory cytokines, including TNF-╬▒ and IL-6, in the ulinastatin-treated group. This negative result complicates the analysis of the well-known causal relationship between CPB-induced systemic inflammation and resulting organ injury and dysfunction during cardiac surgery. CPB produces multiple organ injury and dysfunction; it activates neutrophils and circulating adhesion molecules. These activated neutrophils can cause various organ injuries by adhering to myocytes and the endothelium and by releasing a variety of cell-activating and cytotoxic substances, including potent activators of complements and cytokines, and initial systemic inflammatory triggers such as IL-6, IL-8, TNF-╬▒, and endotoxins [
17-
23]. However, our result corresponded to those of a previous study showing that plasma IL-6 and TNF-╬▒ did not increase after CPB, despite a considerable increase in plasma IL-8. That study also showed a preferential increase of gene expressions for IL-6, IL-8, and TNF-╬▒ in airway spaces (alveolar macrophages) rather than in plasma (plasma monocytes). The post-CPB increase in airway proinflammatory cytokines was up to eight times greater than that in plasma, suggesting that CPB preferentially produces an inflammatory response in the lungs [
24]. Nakanishi et al. [
11] insisted that IL-8 is much more closely related with lung function and can be used to predict post-CPB oxygenation profiles, whereas IL-6 is closely related with myocardial function.
These results may justify the absence of an intergroup difference in the IL-6 level in the present study despite the greater PaO2/FiO2 ratio, and an analysis of cytokines in alveolar macrophages may be required to clarify the efficacy of ulinastatin for attenuating the inflammatory reaction and pulmonary injury.
Bingyang et al. [
15] suggested that a higher dosage (400,000 U) and a longer ulinastatin infusion period are required for better postoperative organ function and reduced release of CPB-induced proinflamatory cytokines. The relatively short half-life of ulinastin (33 min after a 3-h infusion) compared to the duration of CPB (60 min) in this study may have been insufficient to cover the entire CPB period and to attenuate the CPB-induced inflammatory process [
14].
We excluded high-risk patients with more frequent and exaggerated post-CPB myocardial or pulmonary dysfunction in whom the possible efficacy of ulinastatin may be more prominent. To overcome this mismatch and to assess the possible efficacy of ulinastatin for attenuating the CPB-induced release of proinflammatory cytokines, further study using a much higher dosage and longer infusion of ulinastatin is indicated. Furthermore, as the sample size focused on improving the oxygenation profile, it may have been insufficient to speculate the other parameters as secondary outcomes in this study. Thus, additional trials with a much larger sample size are advocative.
In conclusion, CPB pretreatment with ulinastatin resulted in less cardiopulmonary injuries and better cardiac pulmonary performance during the post-CPB period, as indicated by an improved oxygenation profile and reduced inotropic support; however, it did not attenuate the CPB-induced release of proinflammatory cytokines. Additional studies adopting a much higher ulinastatin dosage during the entire period, a larger sample size, and an airway cytokine analysis are necessary to confirm the efficacy of ulinastatin to attenuate the CPB-induced release of inflammatory cytokines and to enhance post-CPB organ function.