Introduction
WIN55212-2 is a synthetic cannabinoid agonist and is selective to the cannabinoid 1 (CB1) receptors, which is distributed mainly in the central nervous system. Opioid receptors and CB1 receptors have several similarities. CB1 receptors, like opioid receptors, are a member of the G protein-coupled receptor (GPCR) family, and their mechanisms of intracellular signal transduction are through coupling to Gi/o proteins, which inhibits adenylate cyclase and decreases cyclic AMP production [1]. Opioid receptors and CB1 receptors are abundantly codistributed in the basal ganglia, caudate, putamen, dorsal hippocampus, amygdala, cerebellum, substantia nigra, nucleus accumbens, periaqueductal grey, and lamina II in the spinal cord [2]. Opioids and cannabinoids also share many pharmacologic properties, including hypothermia, sedation, hypotension, inhibition of intestinal motility, motor depression, antinociception, and reinforcement [3].
The results of the behavioral studies using selective opioid receptor antagonists, administered intracerebroventricularly or intrathecally, have suggested a major involvement of ┬Ą-opioid peptide (MOP) receptors in the supraspinal cannabinoid antinociception [4]. Cross-tolerance was also demonstrated for the cannabinoids and ╬║-opioid peptide (KOP) agonists [5]. The results of in vitro studies suggested that high concentrations of CB1 antagonist act on MOP receptors in a CB1-independent manner [6]. From these shared properties and reports of in vitro and in vivo studies, opioid receptors and CB1 receptors can be cross-regulated, and there may be the possibility of functional links in the mechanisms of their actions.
Such results may suggest an interaction between opioids and cannabinoids at the level of receptor-ligand interactions and intracellular signal transduction mechanisms. Furthermore, antinociception, one of the shared pharmacologic properties of opioids and cannabinoids, particulary attract interest in their interactions. The present study investigated whether CB1 receptor-stimulated [35S]GTP╬│S binding is affected by selective opioid antagonists in the rat brain membranes. We found that activation of G proteins by the CB1 agonist, WIN55212-2, is not blocked by antagonizing the three subtypes of opioid receptors, indicating that there is no direct interaction between these receptors at least at the level of receptor-ligand interaction.
Materials and Methods
Materials
[35S]GTP╬│S (1,250 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA, USA). WIN55212-2 ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate), naloxone (NLX), nor-binaltorphimine (BNI), naltrindole (NTI), GTP╬│S, GDP, and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Membrane preparation
Membranes were isolated from the brains of twenty Sprague-Dawley rats (male), weighing 180-200 g. Rats were maintained in accordance with the University Committee on the Use and Care of Animals and the Guide for the Care and Use of Laboratory Animals. After euthanasia by intravenous thiopental sodium (100 mg/kg), each rat brain was rapidly excised and placed on dry ice before storage at -70Ōäā. Subsequent handling of the tissues was performed at 4Ōäā. Brain tissues were meticulously dissected, washed in 50 mM Tris-HCl buffer (pH 7.4), and then disrupted for 1 min in ice-cold buffer with a Polytron homogenizer, set at a power of 6.5 (model PT-10; Brinkmann Instruments, Westbury, NY, USA). The homogenized membranes were centrifuged at 18,000 g for 15 min. The resulting membrane pellets were resuspended and incubated at 37Ōäā for 40 min to remove endogenous opioids [7]. The preparation was centrifuged again and the pellets resuspended in 50 mM Tris-HCl buffer. Aliquots of this suspension were frozen at -70Ōäā. The protein concentration of the rat brain membrane suspensions was approximately 7-8 mg/ml, as determined by the Bradford assay with bovine serum albumin as the standard [8].
[35S]GTP╬│S binding assay
Agonist stimulation of [35S]GTP╬│S binding by WIN55,212-2 was measured as described in Traynor and Nahorski [9]. Membranes (20-60 ┬Ąg of protein/tube) were incubated in [35S]GTP╬│S binding buffer (20 mM HEPES, pH 7.4, 100 mM NaCl, and 10 mM MgCl2┬Ę6H2O), containing [35S]GTP╬│S (0.1 nM), GDP (100 ┬ĄM), and varying concentrations (3-30,000 nM) of WIN55212-2 in a total volume of 500 ┬Ąl for 60 min at 25Ōäā. Inhibition of agonist-stimulated [35S]GTP╬│S binding by NLX (20 nM), BNI (3 nM), or NTI (3 nM) was evaluated by adding the antagonist to the membrane 15 min before the addition of WIN55212-2. The reaction was terminated by rapidly filtering (Brandel cell harvester, Gaithersburg, MD, USA) through no. 32 glass fiber filters (Schleicher & Schuell, Keene, NH, USA) and washing three times with 2 ml of ice-cold GTP╬│S binding buffer [10]. Filters were placed in scintillation vials with 4 ml of Econo-Safe scintillation cocktail (Beckman Coulter, CA, USA) for liquid scintillation counting (Beckman LC6500, CA, USA). Basal binding was determined from tubes containing the same volume of [35S]GTP╬│S binding buffer without agonist or antagonist. Nonspecific binding was defined as the binding of [35S]GTP╬│S in the presence of 10 ┬ĄM unlabeled GTP╬│S. Because nonspecific binding was less than 5% of basal binding in this condition, the basal count per minute (cpm) was subtracted from each data point and converted to the percent over basal to determine agonist-stimulated [35S]GTP╬│S binding. All rat brain membrane experiments were performed at least twice, and each experiment was performed in duplicate.
Data analysis
[35S]GTP╬│S binding data from two experiments were combined and fit to a sigmoidal curve with a variable slope using GraphPad Prizm (Prism version 4.0, GraphPad Software, Inc., San Diego, CA, USA) to determine the EC50 value and maximum stimulation (% over basal). Mean values (mean ┬▒ SEM) for the maximal stimulation of [35S]GTP╬│S binding and EC50 were calculated from six sets of independent experiments. The Ke values for antagonist inhibition were calculated using the following equation [11]: Ke = [nanomolar antagonist] / (dose ratio-1), where dose ratio is the ratio of the EC50 for WIN55212-2 in the presence and absence of each opioid antagonist.
Results
Optimal stimulation of [35S]GTP╬│S (0.1 nM) binding to the rat brain membranes by WIN55212-2 was observed at a concentration of 100 ┬ĄM GDP (data not shown). The value of mean ┬▒ SEM for the maximal stimulation of [35S]GTP╬│S binding was 27.6 ┬▒ 5.3% over basal in the rat brain membranes (n = 6). The value of EC50 ┬▒ SEM was 154 ┬▒ 39.5 nM (n = 6).
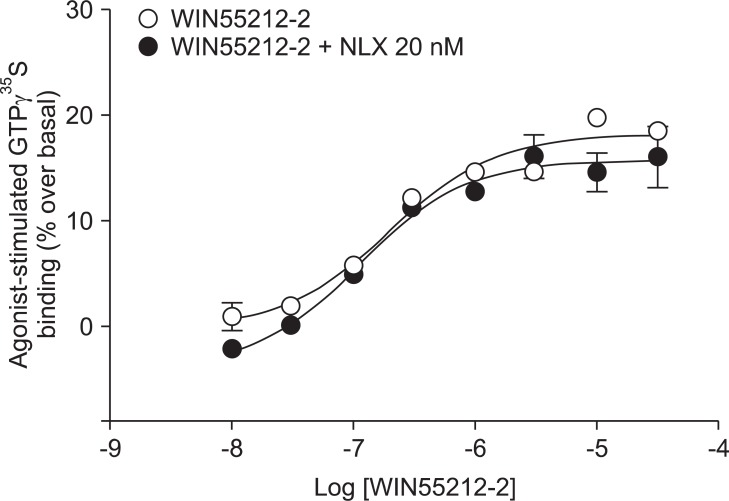
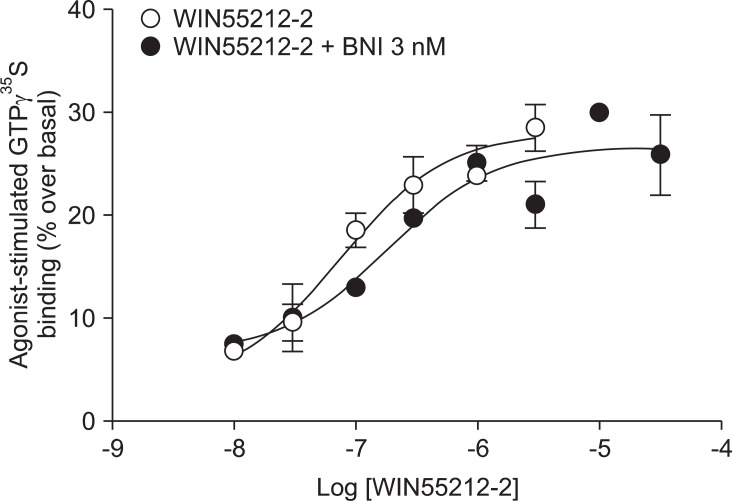
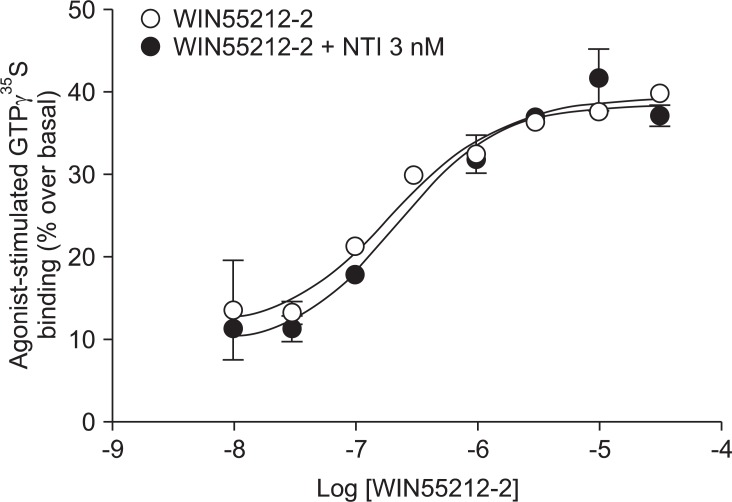
Addition of a selective MOP antagonist at a low concentration (NLX 20 nM) did not produce a rightward shift in the WIN55212-2 concentration-response curve (EC50 = 111 nM, Ke = -46.1; Fig. 1). Addition of a selective KOP antagonist (BNI 3 nM) produced a 2.4-fold rightward shift in the WIN55212-2 concentration-response curve (EC50 = 180 nM, Ke = 2.1; Fig. 2). Addition of a selective ╬┤ opioid peptide (DOP) antagonist (NTI 3 nM) did not produce a rightward shift in the WIN55212-2 concentration-response curve (EC50 = 224 nM, Ke = 17.4; Fig. 3).
Discussion
Similarities in the receptor distributions and pharmacological characteristics between opioids and cannabinoids have been demonstrated. The expression patterns of MOP receptors and CB1 receptors overlap in several areas of the central nervous system. In regions such as caudate, putamen, the dorsal hippocampus, and the nucleus accumbens, MOP and CB1 receptors are co-expressed in the same neurons [12,13]. Thus, possible interactions between opioids and cannabinoids have been suggested, and they have been investigated at receptor-ligand interaction and intracellular signal transduction level [5,6]. It has also been demonstrated that cross-regulation between MOP and CB1 may occur through a direct interaction [14]. However, based on the above evidence, there are additional unidentified receptor-ligand interactions and intracellular signal transduction mechanisms that need to be clarified between the MOP and CB1 receptors.
It was observed that the functional maximal activities of agonist-stimulated [35S]GTP╬│S binding by WIN55212-2 are 27.6 ┬▒ 5.3 % over basal (mean ┬▒ SEM, n = 6). Others have reported that the functional maximal activities are up to 80% over basal [15]. In these studies, however, human embryonic kidney 293 (HEK-293), Neuro-2A, or human neuroblastoma cells (SK-N-SH) were used to prepare the membrane fractions for the [35S]GTP╬│S binding assays [15]. As such, the variations may have come from the different types of the membranes used. The type of membrane denotes the densities of CB1 receptors in specific membranes, the functional power of biologic milieu for the activation of [35S]GTP╬│S binding. The EC50 value (mean ┬▒ SEM) of the concentration-response curve by WIN55212-2 was 154 ┬▒ 39.5 nM, which was consistent with the findings of other studies [15]. Two to three fold higher concentrations of WIN55212-2 were needed for rat brain membranes.
CB1 receptors are well known to be closely related to opioid receptors in terms of antinociception (MOP receptors in particular), and studies of both receptors have revealed the existence of bidirectional cross-tolerance [16,17] and cross-addictive effects between opioids and cannabinoids [18]. Furthermore, CB1 receptors form multimers, as they do in various other G-protein coupled receptors [17]. Although only the homodimeric form of CB1 multimer has been found thus far, CB1 receptor may be a potential partner for association with opioid receptors [19]. In the present study, selective opioid antagonists [NLX (20 nM) as a selective MOP antagonist, BNI (3 nM) as a selective KOP antagonist, and NTI (3 nM) as a selective DOP antagonist] were used to investigate the involvement of opioid receptors at the level of receptor-ligand interaction and intracellular signal transduction. The antagonist results showed no significant rightward shift of the concentration-response curve by WIN55212-2-stimulated [35S]GTP╬│S binding (Fig. 1, 2 and 3). In addition, the Ke values for inhibition of each opioid antagonist were not applicable. When a fixed dose of selective opioid antagonist is used in our experiment with graded doses of WIN55212-2, it is possible to determine the affinity of the antagonist (Ke) for the CB1 receptors [11]. Adding high dose of NLX, in a concentration that nonselectively antagonizes all three opioid receptors (200 nM), did not result in a rightward shift in the concentration-response curve (data not shown).
Taken together, agonist-stimulated [35S]GTP╬│S binding by the CB1 agonist WIN55212-2 was not affected by selective opioid antagonists in the rat brain membranes. Though several studies have described the functional interactions between opioids and cannabinoids at the cellular and behavioral levels, the present results suggest that the functional activity of WIN55212-2 have not been influenced by opioid antagonists at the level of receptor-ligand interaction and intracellular signal transduction. Although complete understanding of the exact mechanism remains unclear, the results may partially elucidate their actions.