Comparison of meperidine and nefopam for prevention of shivering during spinal anesthesia
Article information
Abstract
Background
Shivering is a frequent event during spinal anesthesia and meperidine is a well-known effective drug for prevention and treatment of shivering. Nefopam is a non-opiate analgesic and also known to have an anti-shivering effect. We compared nefopam with meperidine for efficacy of prevention of shivering during spinal anesthesia.
Methods
Sixty five patients, American Society of Anesthesiologists physical status I or II, aged 20-65 years, scheduled for elective orthopedic surgery under spinal anesthesia were investigated. Patients were randomly divided into two groups, meperidine (Group M, n = 33) and nefopam (Group N, n = 32) groups. Group M and N received meperidine 0.4 mg/kg or nefopam 0.15 mg/kg, respectively, in 100 ml of isotonic saline intravenously. All drugs were infused for 15 minutes by a blinded investigator before spinal anesthesia. Blood pressures, heart rates, body temperatures and side effects were checked before and at 15, 30, and 60 minutes after spinal anesthesia.
Results
The incidences and scores of shivering were similar between the two groups. The mean arterial pressures in Group N were maintained higher than in Group M at 15, 30, and 60 minutes after spinal anesthesia. The injection pain was checked in Group N only and its incidence was 15.6%.
Conclusions
We conclude that nefopam can be a good substitute for meperidine for prevention of shivering during spinal anesthesia with more stable hemodynamics, if injection pain is effectively controlled.
Introduction
Shivering associated with spinal anesthesia is a frequent event, and the reported median incidence of shivering related to neuraxial anesthesia is up to 55% [1]. The mechanism of shivering in patients undergoing spinal anesthesia is not clear, but hypothermia due to redistribution of heat caused by vasodilation below the level of a neuraxial block is suggested. Spinal anesthesia also impairs the thermoregulation system by inhibiting vasoconstriction.
Shivering increases oxygen consumption, metabolic rate, lactic acidosis, carbon dioxide production, plasma catecholamines, and cardiac output. Shivering movement may interfere with monitoring of hemodynamics as well as increasing patient discomfort and distress. Therefore, it is very important to prevent shivering during spinal anesthesia.
Many pharmacological trials for prevention or treatment of shivering during spinal anesthesia have been tried by using drugs like fentanyl, meperidine, clonidine, and tramadol, etc [1]. The studies using fentanyl were via epidural or intrathecal injection and there is no study for intravenous injection [1-4]. Ketamine can also decrease shivering during spinal anesthesia, but its property of stimulating sympathetic nervous system induces increasing blood pressure, heart rate, secretion, and hallucination. These make the restrictive use of ketamine [1,5,6]. When clonidine was injected intravenously, shivering was effectively treated, but hypotension and bradycardia occurred [7-9]. There are many studies for meperidine via intravenous, intrathecal, and epidural administrations [1,10,11]. Meperidine was very effective for prevention and treatment of shivering after general and neuraxial anesthesia, but hypotension and drowsiness occurred frequently as the side effects.
Nefopam is a non-opiate, non-sedative analgesic and also known as effective for treatment of shivering after general anesthesia [1,12]. It does not induce respiratory depression and drowsiness. If nefopam is effective for prevention of shivering during spinal anesthesia without side effects, it may be a better substitute for meperidine. Therefore, we compared nefopam with meperidine for preventive effects for shivering, hemodynamics, and side effects during spinal anesthesia.
Materials and Methods
This study was a prospective, double blind, randomized, controlled study. After the approval of the institute's ethics committee and obtaining written informed consents, 65 patients of American Society of Anesthesiologists physical status I or II, aged 20-65 years, undergoing spinal anesthesia for elective orthopedic surgery were investigated. Patients with uncontrolled hypertension, diabetes mellitus, hepatic or pulmonary disease, and fever (temperature > 38℃) were excluded. The surgical procedures included arthroscopic surgery of knee or ankle, hallus valgus correction, total ankle replacement, open tibia osteotomy, hardware removal of lower extremities, excision of benign mass, and tendon repair.
Patients were randomly divided into two groups to receive meperidine (Group M) or nefopam (Group N). Meperidine 0.4 mg/kg or nefopam 0.15 mg/kg was prepared and each drug was mixed into 100 ml isotonic saline. In order to facilitate blinding, the test solution was prepared by an anesthetic nurse who was not involved in the present study. Neither the anesthesiologist nor the patient was aware of the kind of a drug. The study drugs were infused intravenously to the patients for 15 minutes just before spinal anesthesia. The ambient temperatures of the operating rooms were maintained at 22℃ and no active warming for patients was used.
All patients received 5 ml/kg of lactated Ringer's solution before spinal anesthesia without any premedication. Spinal anesthesia was performed with a 25-G Quincke spinal needle between the L3-4 interspinal space in the lateral decubitus position and 11-13 mg of 0.5% hyperbaric bupivacaine was injected intrathecally. Bupivacaine doses, sensory block levels, and durations of surgery were checked, and mean blood pressures, heart rates, and tympanic membrane temperatures were measured before (baseline) and at 15, 30, and 60 min after spinal anesthesia. Sensory block levels were tested by using alcohol swabs every 5 minutes after intrathecal injection to find maximal heights of sensory block. Also, sedation scores and shivering scores were checked till 60 min after spinal anesthesia. Sedation score was graded using the Ramsay sedation scale, and shivering score was graded as; 0 = no shivering, 1 = slightly shivering, 2 = muscle shivering, and 3 = severe muscle shivering. Side effects, such as hypotension, nausea and vomiting, pruritus, respiratory depression (SpO2 < 95), and injection pain were recorded. Hypotension was defined as a decrease in mean blood pressure of more than 20% from the baseline or below 60 mmHg, and checked and treated with ephedrine 4 mg repeatedly if occurred.
All statistical analysis was performed with PASW® statistics (version 18 SPSS, Chicago, IL, USA). Demographic data, bupivacaine doses, durations of surgery, sensory block levels, mean arterial pressures, heart rates, and tympanic membrane temperatures between the groups were compared using the Student's t-test. The incidences of side effects and scores of shivering and sedation scales were analyzed with the chi-square test and Fisher's exact test. Correlation among variables was analyzed with Pearson's correlation coefficient. P value of <0.05 was considered as statistically significant.
Results
There was no difference between the groups with respect to demographic data, patients' characteristics related to spinal anesthesia, bupivacaine doses, durations of surgery and sensory block levels (Table 1).
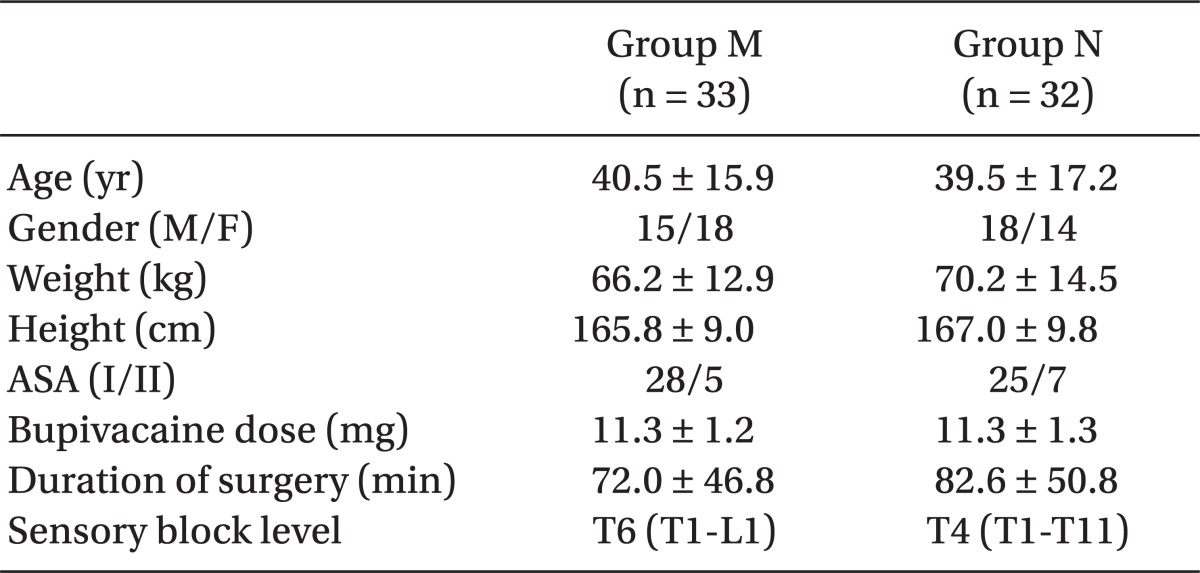
Demographic Data and Patients' Characteristics Related to spinal anesthesia (P = 0.001, 0.002, and 0.010 for mean arterial Spinal Anesthesia
The mean arterial pressures in Group N were maintained higher than in Group M at 15, 30, and 60 minutes after spinal anesthesia (P = 0.001, 0.014, and 0.026 at 15, 30, and 60 minutes, respectively, after spinal anesthesia, Fig. 1). The mean arterial pressures and heart rates in Group M were lower than the baseline values of each parameter at 15, 30, and 60 minutes after spinal anesthesia (P = 0.001, 0.002, and 0.010 for mean arterial pressures, and P = 0.031, 0.003, and 0.007 for heart rates, at 15, 30, and 60 minutes, respectively, after spinal anesthesia, Fig. 1). The tympanic membrane temperatures were not different between the groups except at 60 minutes after spinal anesthesia (Fig. 1).
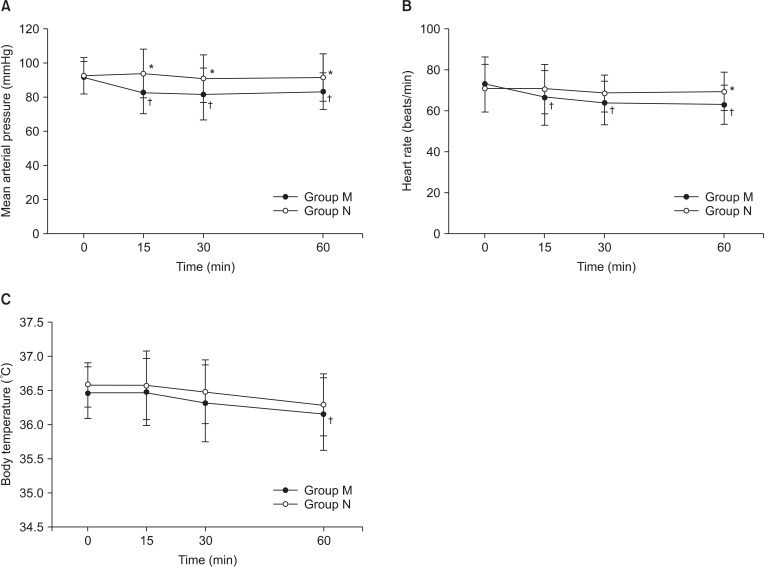
Change of hemodynamics and body temperature. (A) mean arterial pressure, (B) heart rate, and (C) tympanic membrane temperature. Group M: meperidine group, Group N: nafopam group. *P < 0.05, compared to Group M at the same times. †P < 0.05, compared to the baseline value of the same group.
There was no hypotension or vomiting in both groups. The sedation scores, shivering scores, and the incidences of nausea, respiratory depression and pruritus were not different between the groups (Table 2). Although not statistically significant, the incidence of nausea in Group M was much higher than in Group N (7/33 vs. 1/32, P = 0.054) and it was not correlated with sensory block levels (P = 0.778). Injection pain was observed in Group N only and its incidence was 15.6% (P = 0.024, Table 2).
Discussion
Nefopam is a non-opioid, non-steroidal centrally-acting analgesic. Its chemical class is benzoxazocine which is structually related to diphenhydramine (an antihistamine drug) and orphenadrine (an antimuscarinic drug). It is used mainly in European countries for the relief of moderate to severe pain as an alternative to opioid analgesics. Its mechanism underlying the pharmacological actions is not clear, but it is suggested that the inhibition of synaptosomal uptake of serotonin, norepinephrine and dopamine is related to its analgesic properties [13-17]. Nefopam also directly interacts with α2-adrenoceptors [14] and is a noncompetitive NMDA receptor antagonist [18]. Nefopam has an additional action of the prevention of shivering and it is related to inhibition of monoamine and NMDA receptors.
Because nefopam does not produce respiratory depression, sedation and hypotension, it was usually studied for analgesics in critically ill patients [19] or for prevention of postoperative shivering due to hypothermia [20-22]. Bilotta et al. [12] first reported that nefopam was superior to tramadol for prevention of shivering during neuraxial anesthesia. Our study is also the first in comparing nefopam with meperidine for prevention of shivering during spinal anesthesia. In our study, the prophylactic administration of nefopam reduced the incidences and scores of shivering during spinal anesthesia similar to meperidine.
In the study of Bilotta et al. [12], there was no difference between nefopam and tramadol in hemodynamics. However, in our study, nefopam maintained blood pressure higher compared with meperidine. While most thermoregulatory drugs including meperidine reduce both the thresholds of shivering and vasoconstriction, Alfonsi et al. [23] reported that nefopam significantly reduced only the shivering threshold. These are clinically important because thermoregulatory vasodilation can induce reduction of nearly 20 mmHg in mean arterial pressure. Maintaining higher blood pressure may be due to the property of nefopam sparing vasoconstriction threshold.
Another advantage of nefopam is that nefopam does not induce respiratory depression. In this study, there was no case of respiratory depression in the nefopam group same as in the meperidine group. It means that nefopam can be used safely for prevention of shivering during spinal anesthesia in critically ill patients with hemodynamic instability because the risks of repiratory depression together with sedation and hypotension can be excluded or minimized. Especially, it is expected that nefopam can reduce severe postoperative morbidities such as myocardial infarction by preventing the increase of oxygen consumption due to shivering.
Common side effects of nefopam are sweating, nausea, tachycardia and pain at injection, etc [20,24,25]. However, in our study, there was no sweating or tachycardia. Although the incidence of nausea was not different between the two groups statistically, nausea was more frequently observed in the meperidine group than in the nefopam group and it was not correlated with the sensory block levels. Pain at injection was observed in the nefopam group only and it is considered to be related to the rapid infusion of nefopam. It resolved spontaneously within 30 minutes in all cases and this may suggest that the injection pain is associated with rapid increases in cerebral concentration of nefopam [20].
The limitations of this study are three. First, we used the duration of 15 minutes for infusion of meperidine or nefopam. The usual recommendation for intravenous administration of nefopam is slow infusion over 15 minutes or more. Moreover, the dilution of the nefopam with 100 ml of isotononic saline was thought to be sufficient to prevent the injection pain by tight control of slow infusion. However, in our study, five patients complained of pain at injection of nefopam on their limbs with IV access. Thus, it is thought that the injection pain can be prevented or reduced by slower infusion of nefopam. Second, the overall sensory block levels in the two groups were high. It may produce an unexpected bias in the incidences of side effects. Third, the study duration for each subject was short as 60 minutes. Actually, we focused on the effect of nefopam to prevent shivering by spinal anesthesia itself because the kinds and duration of surgery may affect the core temperature by cooling the body. So, we chose the study duration of 60 minutes. However, it is thought that the incidence and degree of shivering during the intraoperative and postoperative period is also meaningful because the presence or absence of shivering is more important for a patient.
In summary, nefopam demonstrated a preventive effect for shivering during spinal anesthesia similar as meperidine. However, nefopam has significant injection pain, presumed to be due to rapid infusion. If we can determine the infusion rate of nefopam which will not induce injection pain, it will be more helpful and comfortable to conscious patients requiring the prevention or treatment of shivering. Therefore, further investigations to reduce or prevent injection pain of nefopam are needed. Nefopam maintained mean arterial pressures more stable than meperidine. It implies that nefopam can be a good substitute for meperidine for prevention of shivering during spinal anesthesia in hemodynamically unstable patients, if injection pain is effectively controlled.
Acknowledgments
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2007 (6-2007-0175).
