|
 |
|
|
Abstract
Background
There are a number of adjuvants to be used for local anesthetics in spinal block. The aim of this study was to demonstrate the possible effect of intrathecal midazolam compared with bupivacaine as adjuvants in spinal anesthesia with bupivacaine in chronic opium abuses.
Methods
In a double blind, randomized clinical trial, 90 opium abuser patients undergoing lower limb orthopedic surgery were selected and randomly assigned into 3 groups (30 cases each). The patients received 15 mg plain bupivacaine, or 15 mg bupivacaine plus 25 mcg fentanyl or 15 mg bupivacaine plus 1 mg midazolam, intrathecally.
Opioid agonists are one of the most common therapeutic modalities to relieve pain [1,2]. However, these compounds when used in a prolonged manner would produce an increasing demand state [3,4]; which is a state of increasing need for producing the same effects; so, it would be accompanied by an elevation of the consumption dose to get a constant analgesic effect; which is known as analgesic tolerance [5,6]. A number of studies have proposed new insights into pain [6,7] and its regulation in opium abusers [7,8]. Usually, acute pain suppression is accompanied with many more problems in opium abuser patients [9], a state known as opium tolerance [10,11].
In chronic opium abusers, there are a number of previous studies that have demonstrated a shorter duration of neural block after intrathecal administration of local anesthetics for spinal anesthesia; which is longer than non abusing patients [12,13]. However, the mechanism underlying this clinical phenomenon has not been yet fully clarified [14,15].
However, opium abusers are very extraordinary clinical pain models that demonstrate altered pain states; using adjuvant agents could uncover parts of their underlying chronic pain mechanisms that would possibly help us discover more aspects of chronic pain that require acute pain remedies; this would be especially beneficial in the treatment of opium abusers.
This study was conducted to compare the effect of midazolam and fentanyl on the duration of spinal anesthesia with 0.5% bupivacaine in opium abusers undergoing lower limb orthopedic surgery, to see which compound would have a better role as the adjuvant drug in spinal anesthesia with bupivacaine in opium abusing patients.
The proposal of the study was approved by the IRB Committee, Research Deputy, Department of Anesthesiology, Faculty of Medicine, Shahid Beheshti University of Medicine, Tehran, Iran, regarding the ethical concerns for human trials.
In a double blind, randomized clinical trial, the target population was considered as entirety of all the surgical patients being admitted to the operating room of the hospital, which was the location of the study, during a 12 months period.
Sample size determination was done through a power analysis using the Power Analysis and Sample Size software: PASS 2005 (alpha = 0.02, beta = 0.2 and power = 0.8). In this study, our clinical criterion for determination of the sample size was the observed frequency of spinal sensory block duration. Normal distribution of the data was also controlled.
After an informed written consent, 90 patients aged 18-65 years and heights of 150-185 centimeters who were scheduled for elective lower limb surgery, were randomly divided into three groups, provided with a closed box, having 90 labels in it. The box contained 30 B labels having the letter B on it standing for Bupivacaine alone, 30 BF labels standing for Bupivacaine plus Fentanyl and 30 BM labels standing for Bupivacaine plus Midazolam. Random allocation of clinical cases was done through blind label taking for each case from this box. The first group (Group B) received 15 mg plain preservative free bupivacaine intrathecally plus 1 ml sterile normal saline to have a 4 ml solution, the 2nd group (Group BF) received 15 mg preservative free bupivacaine plus 25 mcg fentanyl intrathecally and the 3rd group (Group BM) received 15 mg preservative free bupivacaine plus 1 mg preservative free midazolam intrathecally. The total volume of injection fluid was similar in the 3 groups (4 ml in each group); since this study was done double blindly.
All the participants were chronic opium abusers, who used opium preparations orally or by an inhalation route, and this pattern was a regular habit, with durations of at least two years. In addition, the patients had experienced subjective symptoms of withdrawal whenever drug cessation occurred. Exclusion criteria were: patient refusal of subarachnoid block; abuse or illicit use of other controlled drugs or substances, pre-existing cardiac or pulmonary disease, or any sign or clinical finding denoting past or present neuropathy.
The night before the surgery, all patients were visited by the same anesthesiologist (from among the authors); during this visit, they were informed about the study. Also, the anesthesiologist prescribed a pre-medication dose of promethazine for a 0.05 mg/kg intramuscular administration 1 hour before the surgery; also, a 10 mg diazepam tablet per 70 kg body weight was advised for the night before the surgery. Meanwhile, the patients were recommended to use their usual daily opium dose in order not to create an acute episode of pain due to withdrawal of the opioid compounds, although the stop, skip of daily opium is more similar to usual clinical situations in hospitals.
The anesthesiologist who had visited the patient the evening prior to surgery discussed the issue in a private room from each patient. All the patients were NPO for eight hours before the scheduled surgical procedure.
Inside the operating room, the anesthesiologist who performed the subarachnoid block and documented the sensory level was blinded to the patient's group. The standard monitoring (including ECG, pulse oxymetry, non-invasive blood pressure and heart rate) were initiated first; then each patient received 500 ml of Ringer's solution over 15-20 minutes. Afterwards, subarachnoid drug administration was performed with the patient in the sitting position under appropriate aseptic conditions with a guardian nurse. The L3/4 interspace was entered and a 25 gauge Whitacre spinal needle was inserted via a midline approach using a cephalad bevel method; while 4 ml of sterile preservative free solution was injected at a rate of 2 ml every 5 seconds (in group B: 15 mg bupivacaine plus 1 ml normal saline; in group BF: 15 mg bupivacaine plus 25 mcg fentanyl; in group BM: 15 mg bupivacaine plus 1 mg midazolam). The bupivacaine used in this study was 0.5% plain preservative bupivacaine with isobaric properties (Sensorcaine, Astra Zeneca, UK).
After drug injection, the patients were placed supine. Using position maneuvers after intrathecal administration of drug, a T8 to T10 level of anesthesia was achieved. Using a pinprick test, sensory level was assessed each minute thereafter for 10 minutes. Then, the anesthesia level was checked and documented in minutes 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140 and 150 after subarachnoid drug administration.
In case of the slightest pain sensation for any patient at any time during the operation, general anesthesia was induced immediately and the subarachnoid effects of the local anesthetic drug (spinal anesthesia) were considered to be terminated; then, the exact final time of spinal anesthesia was recorded. The total time for effective spinal anesthesia was recorded from the drug injection time (as the start point) up to the time that a 2 segment regression in the level of block (using a pinprick test) was detected. Also, if the intrathecal drug administration failed to create a documented sensory block, the case was deleted and the patient was replaced with another.
For postoperative analgesia, intravenous morphine was administered to keep the patients pain recording below 3 of 10 based on a 10 steps visual analog scale (VAS). The total postoperative morphine requirements during the first 24 hours were recorded and compared between the 3 groups.
SPSS software (version 11.5) was used for data entry and analysis. Also, ANOVA and post hoc analysis were used as the statistical tests for data analysis and a P value less than 0.05 was considered significant.
Spinal anesthesia failed in two patients from group B, 3 from group BF and only one patient from group BM. They were excluded from the study, and then new cases replaced them.
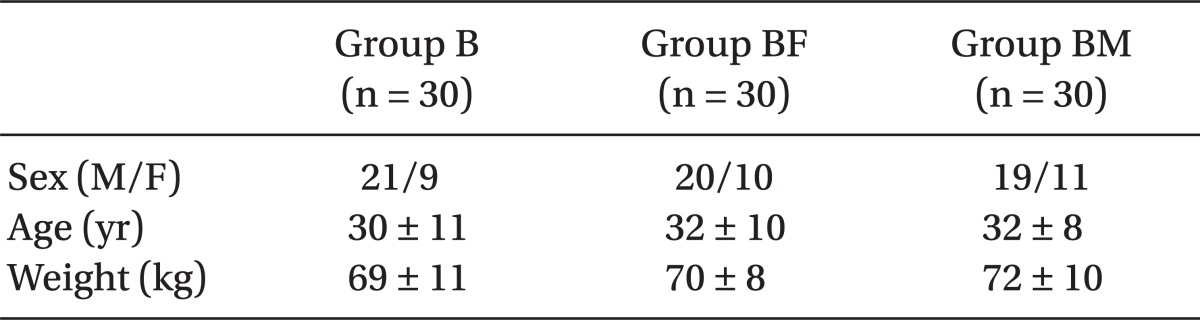
The three groups had no significant difference regarding baseline characteristics including age, body weight, gender and surgical duration (Table 1). As mentioned in Table 2, in group B, the duration of sensory block was the lowest, then group BF, and finally group BM had the highest length of sensory block; (P = 0.02). This difference was statistically significant among the three groups.
Also, Post Hoc Tukey test (Table 2) demonstrated a significant difference between group BF and group BM (P = 0.02); also, the difference between B and BM groups was statistically significant according to the Post Hoc Tukey test (P = 0.001). The same was true regarding the difference between B and BF groups (P = 0.02).
There was a statistically significant difference among the 3 groups regarding 24 hours postoperative morphine requirements (Table 2); group B was the highest, then group BF, and finally group BM needed the lowest morphine (P = 0.04).
This study demonstrated increased duration of sensory block after subarachnoid injection of adjuvant midazolam or fentanyl to plain 0.5% bupivacaine in opium abusers undergoing lower limb orthopedic surgery; the effect of adjuvant midazolam was more pronounced than fentanyl in these cases.
Asking people about their previous opium abuse was not such an easy task to do. Also, there was no possibility to objectively document the opium abuse history in the cases without any interference in the ethical considerations of the study. This is one of the limitations of the study.
Also, all the patients in the study were recommended to take the usual daily opium dose: however, there was not the possibility to compare the exact dose of opium in the abused material, since the abused material does not have any standardization and is used in "illegal" or "informal" forms. So, the exact amount of the dose could not be described, and also, there is a possibility that some patients might not have taken the usual daily opium; therefore, this is another limitation of the study.
Had there been a calculation of the exact motor blockade time, a more accurate interpretation of the block properties could have been possible; without the limitations of the surgical procedure.
The type of local anesthetic, drug dosage, drug adjuvants like opioids [15], epinephrine [4] and intravenous drugs [16] are among the items named to be affecting the duration of subarachnoid block in spinal anesthesia. It has been demonstrated that opium abusers have a shortened duration of block compared with non-abusers in patients undergoing subarachnoid lidocaine [12] or bupivacaine [13] injection; a number of mechanisms have been proposed, but the which one is not fully clear yet.
In one study, it was demonstrated that in patients undergoing cesarean delivery, 2 mg intrathecal midazolam, when used as an adjunct to bupivacaine, could provide a moderate prolongation of postoperative analgesia; also, this study demonstrated that 1 and 2 mg intrathecal midazolam could decrease postoperative nausea and vomiting [16]. It has also been demonstrated that intrathecal midazolam appears to improve perioperative analgesia and reduce nausea and vomiting during caesarean delivery [17]; the intrathecal effects of midazolam have been proposed to be due to its intrathecal spinal receptor interactions [18], suggesting the drug to be affecting the type A receptors of the gamma-aminobutyric acid (GABA), a role which has a very important effect in antinociceptive mechanisms [19,20] in the spinal cord neurons [21,22]. The present study demonstrated prolongation of the effects of intrathecal bupivacaine after adding midazolam as an adjuvant in these cases - more than the effects of intrathecal adjuvant fentanyl. This finding will be assessed more rigorously, from consideration in the future research, after having a detailed examination at the spinal receptors like GABA, as proposed in the previous studies.
But, the effects of the findings in this study are somewhat different. As a matter of fact, the altered and "non-physiologic" pain perception in opioid abusers is a pain model with an altered response to analgesic medications. The assessment of pain responses in opium abusers helps us understand more about the pain interlay of the spine.
Why is the effect of adjuvant midazolam more pronounced than fentanyl in opium abusers? There is not an approved and documented response available for this finding; however, there may be some theoretical explanations. The most probable explanation is that the patients with a history of opium abuse have chronic exposure to opioid compounds in such a way that the repeated exposure has created a down-regulation of response to opioids. It is a fact that chronic administration of morphine can induce desensitization of the spinal cord receptors to morphine in rats [23,24]; in addition, it has been shown that chronic morphine use cause down regulation of spinal glutamate transporters which is accompanied with abnormal pain sensitivity [25].
The findings of this study demonstrated increased duration of sensory block after subarachnoid injection of adjuvant midazolam or fentanyl to plain 0.5% bupivacaine in opium abusers undergoing lower limb orthopedic surgery; the effect of adjuvant midazolam was more pronounced than fentanyl in these cases. This may help in selecting adjuvant drugs for spinal bupivacaine administration in these cases to prolong spinal blockade.
There are studies that have demonstrated subarachnoid injection of adjuvant midazolam or fentanyl to bupivacaine could increase the duration of sensory block in non-abusers. However, the final conclusion of this study was different from the others regarding the assessment of sensory and motor block in opium abuser patients.
Chronic opioid abuse would alter the pain mechanisms of the spinal cord, leading to newly emerged chronic pain syndromes and poorly controlled acute pain states after surgery. Therefore, as far as we know, opium abusers have a comprehensive body tolerance to opioids; this tolerance appears not only for opioids but also for local anesthetics; both in clinical [12,13] and animal models [22] with a poorly defined mechanism.
However, the effects of midazolam or fentanyl on the duration of spinal block by bupivacaine could be a new finding. Spinal cord receptors of midazolam are not affected by chronic opium abuse or at least, are affected less than spinal receptors of opioids and local anesthetics in chronic opium abusers. This finding could introduce us to create similar animal models to compare the spinal receptors of opioids, local anesthetics and benzodiazepines, in order to find some new therapeutic remedies for these patients which in turn could control neuropathic pain states.
This issue would be even more interesting when we compare the effectiveness of the bupivacaine-midazolam combination compared to the bupivacaine-fentanyl combination in opium abusers with non abusers: the results are really controversial in non-abusers! This is the important finding that would help us understand more underlying pain mechanisms in the spinal cord. As mentioned, the altered pain perception in opioid abusers creates an alternative pain study model. In non-abusers, the measured duration of spinal anesthesia of adjuvant midazolam with bupivacaine is roughly 115.8-391.6 minutes [26,27] while it is about 116.4-280 minutes in adjuvant fentanyl with bupivacaine patients [28,29].
Subarachnoid injection of adjuvant midazolam or fentanyl to plain 0.5% bupivacaine in opium abusers undergoing lower limb orthopedic surgery increases the duration of sensory block; the effect of adjuvant midazolam is more pronounced than fentanyl in such cases. Though the effects of subarachnoid midazolam or fentanyl to bupivacaine have been demonstrated in non-abuser patients, there were different results regarding opium abuser patients.
Acknowledgments
The authors wish to thank the administrative support of "Deputy of Research Affairs, Anesthesiology Department, Faculty of Medicine, Shahid Beheshti University of Medicine, Tehran, Iran" in the course of the project. Also, the operating room physicians (including their anesthesiology department physicians, especially Dr Alireza Salimi, from Loghman Hospital) and nurses of Loghman and Taleghani Hospitals, Shahid Beheshti University of Medicine, Tehran, Iran are highly appreciated, who provided support for the project and also attended to the patients throughout their treatment in the operating room.
References
1. Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 2005; 80: 319-324. PMID: 15795927.


2. Gardell LR, King T, Ossipov MH, Rice KC, Lai J, Vanderah TW, et al. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci Lett 2006; 396: 44-49. PMID: 16343768.


3. Ossipov MH, Lai J, King T, Vanderah TW, Malan TP Jr, Hruby VJ, et al. Antinociceptive and nociceptive actions of opioids. J Neurobiol 2004; 61: 126-148. PMID: 15362157.


4. Szeto HH, Soong Y, Wu D, Qian X, Zhao GM. Endogenous opioid peptides contribute to antinociceptive potency of intrathecal [Dmt1] DALDA. J Pharmacol Exp Ther 2003; 305: 696-702. PMID: 12606628.


5. Christie MJ, Williams JT, North RA. Cellular mechanisms of opioid tolerance: studies in simple brain neurons. Mol Pharmacol 1987; 32: 633-638. PMID: 2824980.

6. Bovill JG. Mechanisms of actions of opioids and non-steroidal anti-inflammatory drugs. Eur J Anaesthesiol Suppl 1997; 15: 9-15. PMID: 9202932.


7. Wu HE, Thompson J, Sun HS, Leitermann RJ, Fujimoto JM, Tseng LF. Nonopioidergic mechanism mediating morphine-induced antianalgesia in the mouse spinal cord. J Pharmacol Exp Ther 2004; 310: 240-246. PMID: 14999057.


8. Chen SR, Pan HL. Antinociceptive effect of morphine, but not mu opioid receptor number, is attenuated in the spinal cord of diabetic rats. Anesthesiology 2003; 99: 1409-1414. PMID: 14639157.


9. Marchand F, Ardid D, Chapuy E, Alloui A, Jourdan D, Eschalier A. Evidence for an involvement of supraspinal delta- and spinal mu-opioid receptors in the antihyperalgesic effect of chronically administered clomipramine in mononeuropathic rats. J Pharmacol Exp Ther 2003; 307: 268-274. PMID: 12954814.


10. Hurley RW, Banfor P, Hammond DL. Spinal pharmacology of antinociception produced by microinjection of mu or delta opioid receptor agonists in the ventromedial medulla of the rat. Neuroscience 2003; 118: 789-796. PMID: 12710986.


11. Yoburn BC, Gomes BA, Rajashekara V, Patel C, Patel M. Role of G(i)alpha2-protein in opioid tolerance and mu-opioid receptor downregulation in vivo. Synapse 2003; 47: 109-116. PMID: 12454948.


12. Vosoughian M, Dabbagh A, Rajaei S, Maftuh H. The duration of spinal anesthesia with 5% lidocaine in chronic opium abusers compared with nonabusers. Anesth Analg 2007; 105: 531-533. PMID: 17646519.


13. Dabbagh A, Dahi-Taleghani M, Elyasi H, Vosoughian M, Malek B, Rajaei S, et al. Duration of spinal anesthesia with bupivacaine in chronic opium abusers undergoing lower extremity orthopedic surgery. Arch Iran Med 2007; 10: 316-320. PMID: 17604467.

14. Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104: 570-587. PMID: 16508405.


15. Wu Z, Kong M, Wang N, Finlayson RJ, De Tran QH. Intravenous butorphanol administration reduces intrathecal morphine-induced pruritus after cesarean delivery: a randomized, double-blind, placebo-controlled study. J Anesth 2012; 26: 752-757. PMID: 22674156.


16. Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulfate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand 2009; 53: 1088-1091. PMID: 19519724.


17. Prakash S, Joshi N, Gogia AR, Prakash S, Singh R. Analgesic efficacy of two doses of intrathecal midazolam with bupivacaine in patients undergoing cesarean delivery. Reg Anesth Pain Med 2006; 31: 221-226. PMID: 16701187.


18. Ho KM, Ismail H. Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis. Anaesth Intensive Care 2008; 36: 365-373. PMID: 18564797.


19. Nishiyama T. Interaction between midazolam and epibatidine in spinally mediated antinociception in rats. J Anesth 2009; 23: 370-377. PMID: 19685117.


20. Nishiyama T. Interaction between midazolam and serotonin in spinally mediated antinociception in rats. J Anesth 2009; 23: 249-255. PMID: 19444565.


21. Kim HJ, Seol TK, Lee HJ, Yaksh TL, Jun JH. The effect of intrathecal mu, delta, kappa, and alpha-2 agonists on thermal hyperalgesia induced by mild burn on hind paw in rats. J Anesth 2011; 25: 884-891. PMID: 21983967.


22. Dabbagh A, Moghadam SF, Rajaei S, Mansouri Z, Manaheji HS. Can repeated exposure to morphine change the spinal analgesic effects of lidocaine in rat? J Res Med Sci 2011; 16: 1361-1365. PMID: 22973332.


23. Maher CE, Eisenach JC, Pan HL, Xiao R, Childers SR. Chronic intrathecal morphine administration produces homologous mu receptor/G-protein desensitization specifically in spinal cord. Brain Res 2001; 895: 1-8. PMID: 11259753.


24. Tarasiuk A, Gibbs L, Kendig JJ. Descending inhibition in neonatal rat spinal cord: actions of pentobarbital and morphine. Brain Res Bull 1996; 41: 39-45. PMID: 8883914.


25. Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 2002; 22: 8312-8323. PMID: 12223586.



26. Joshi SA, Khadke VV, Subhedar RD, Patil AW, Motghare VM. Comparative evaluation of intrathecal midazolam and low dose clonidine: Efficacy, safety and duration of analgesia. A randomized, double blind, prospective clinical trial. Indian J Pharmacol 2012; 44: 357-361. PMID: 22701246.



27. Kim MH, Lee YM. Intrathecal midazolam increases the analgesic effects of spinal blockade with bupivacaine in patients undergoing haemorrhoidectomy. Br J Anaesth 2001; 86: 77-79. PMID: 11575414.



28. Khezri MB, Yaghobi S, Hajikhani M, Asefzadeh S. Comparison of postoperative analgesic effect of intrathecal magnesium and fentanyl added to bupivacaine in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Taiwan 2012; 50: 19-24. PMID: 22500909.


29. Black AS, Newcombe GN, Plummer JL, McLeod DH, Martin DK. Spinal anaesthesia for ambulatory arthroscopic surgery of the knee: a comparison of low-dose prilocaine and fentanyl with bupivacaine and fentanyl. Br J Anaesth 2011; 106: 183-188. PMID: 20947591.



- TOOLS