|
 |
|
|
Abstract
Pulmonary thromboembolism is one of the most important causes of morbidity and mortality in patients undergoing lower extremity orthopedic surgery. Early diagnosis and appropriate management are important clinical challenges. In this case, massive pulmonary embolism causing sudden cardiac arrest was attributed to use of tourniquet inflation during lower extremity orthopedic surgery. Resuscitation procedures were initiated and transesophageal echocardiography revealed pulmonary thromboembolism. Patients with high suspicion for the presence of deep vein thrombus must be monitored thoroughly during limb exsanguinations.
Pulmonary thromboembolism (PE) is a blockage of the main artery of the lung or one of its branches by blood clots, fat, amniotic fluid, air or a substance that traveled through the bloodstream from another part of the body. Usually this is due to embolism of a thrombus from the deep veins in the legs that can lead to serious complications during the perioperative period [1]. Enforced bed rest and immobility during orthopedic treatment and late operative fixation result in a high incidence of PE, with an even higher incidence associated with compound fractures. We report a case of a patient who developed sudden hypotension and cardiac arrest after tourniquet inflation. Cardiopulmonary resuscitation procedures were instituted successfully and transesophageal echocardiography (TEE) revealed PE.
A 63-year-old, 164 cm, 79 kg male was admitted after sustaining left hip and tibia fracture due to a road traffic accident. The patient underwent a transsphenoidal approach operation due to a pituitary adenoma 5 years previously and had been followed up for panhypopituitarism and diabetes mellitus at our institution.
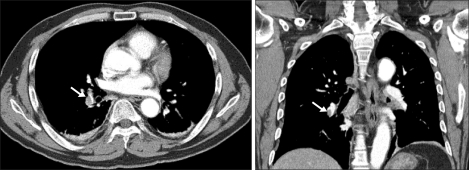
The patient complained of mild dyspnea 10 hours after the traffic accident. Arterial blood gas analysis (ABGA) revealed pH 7.423, PaCO2 37.3 mmHg, PaO2 50.1 mmHg, SaO2 86.5% (FiO2 0.2). The alveolar-arterial oxygen gradient was increased to 53 but his chest x-ray was normal. On the 3rd hospital day, dyspnea and hypoxemia worsened and chest computed tomography (CT) was performed due to suspected PE. Chest CT scan showed thromboembolism in the right interlobar pulmonary artery, right lower lobe segmental artery and subsegmental pulmonary artery (Fig. 1). Heparin treatment was initiated immediately and dyspnea and hypoxemia improved. Transthoracic echocardiography was performed on the 4th hospital day without showing any abnormality or thrombus. On the 7th hospital day, left lower limb venography was taken but there was no venous thrombus and an inferior vena cava filter was not inserted. The patient was scheduled for an operation 11 days after the accident.
During heparin administration activated partial thromboplastin time (aPTT) was maintained between 60 and 67 sec and heparin was stopped at midnight on the day before the operation. Prothrombin time and aPTT were within normal range on the day of operation. Laboratory tests were within normal range except BUN/Cr which was increased to 23.7/1.5 mg/dl.
The patient was not premedicated. On arrival in the operating room, standard monitoring devices were applied. After Allen's test, the left radial artery was cannulated for continuous blood pressure monitoring and the initial blood pressure (BP) was 120/70 mmHg. 5 L of 100% O2 was applied to the patient via oxygen mask and initial ABGA showed pH 7.43, PaCO2 38.1 mmHg, PaO2 102.9 mmHg, and SaO2 98%. Spinal anesthesia was administered with the patient in the right lateral position using a 25-gauge Quincke spinal needle at the level of the L3-4 interspace under standard aseptic conditions. A total of 12 mg of 0.5% tetracaine, mixed with saline, the patient's CSF, and epinephrine 1 : 200,000 was administered slowly to the patient. The patient was then positioned supine and a sensory block to T10 was confirmed by pin-prick test 5 min later. During spinal anesthesia, 700 ml of Hartmann's solution was administered to the patient and there were no significant changes in BP or heart rate (HR). Forty min after spinal anesthesia, a pneumatic tourniquet was inflated on the left thigh. It was inflated to 350 mmHg, without prior use of an Esmarch bandage. Immediately after the skin incision was made, the patient became unresponsive with BP 50/30 mmHg and HR 40 bpm. Ephedrine hydrochloride 8 mg was administered intravenously followed by another 12 mg but BP did not increase. Phenylephrine 300 µg was administered and endotracheal intubation was performed immediately and the patient was ventilated with 100% oxygen. BP increased to 80/70 mmHg but cardiac arrest then followed, and external cardiac massage was initiated. The patient received epinephrine 1 mg and atropine 0.5 mg repeatedly for 4 times without any response and infusion of epinephrine 0.1 µg/kg/min and norepinephrine 0.1 µg/kg/min was started. Fifteen min after cardiopulmonary resuscitation was instituted, HR increased to 140 bpm and BP increased to 140/90 mmHg. ABGA at that time showed pH 7.127, PaCO2 37.2 mmHg, PaO2 144.4 mmHg, HCO3 12.0 mmol/L, BE -15.9 mmol/L, and SaO2 98.1%. Sodium bicarbonate 200 mEq was intravenously administered because of metabolic acidosis. BP was maintained by continuously infusing epinephrine and norepinephrine. Emergent TEE revealed multiple emboli in the right atrium and the interatrial septum to be bulging into the left atrium (Fig. 2). After the TEE diagnosis of PE, vital signs improved (BP 120/70 mmHg, HR 110 bpm) and the operation resumed. The patient was transferred to the intensive care unit (ICU) with 100% oxygen applied through an endotracheal tube (anesthesia and operation time were 135 min and 50 min in length, respectively). On admission to the ICU, mechanical ventilation was continued with FiO2 1.0. ABGA showed pH 7.165, PaCO2 66.6 mmHg, PaO2 66.6 mmHg, and SaO2 87.1%.
Heparin treatment was initiated immediately on arrival to ICU and D-dimer was 9467 ng/ml. D-dimer increased to 52,796 ng/ml on postoperative day (POD) 2. PaO2 continuously improved and ABGA on POD 7 was pH 7.485, PaCO2 32.4 mmHg, PaO2 142.2 mmHg, and SaO2 99.0% (FiO2 0.4). The patient was extubated on POD 9. The patient was on heparin treatment for 20 days. D-dimer started to decrease and chest CT and echocardiography did not show any pulmonary or intracardiac emboli. The patient's vital signs came to within normal range and the patient was transferred to the general ward on POD 30.
PE is a condition that occurs when one or more arteries in lungs become blocked by blood clots, fat, amniotic fluid, air, tumor cells, or, most commonly, from dislodged thrombi originating in the leg veins traveling through the bloodstream to the lung. The obstruction of blood flow through the lungs and the resultant pressure on the right ventricle of the heart leads to serious complications during the perioperative period [1], with the hypercoagulable state of the patient representing a risk factor for PE. The primary risk factors for PE include deficiencies of antithrombin III, proteins C or S and the secondary risk factors include deep vein thrombus or PE history, immobilization, pregnancy, lower limb fracture, heart failure, obesity, cancer, operation lasting longer than 30 min and those older than 40 yr of age [2]. Also, there are reports of PE development during leg elevation or leg manipulation [2,3].
Symptoms and signs of PE include shortness of breath, rapid respiratory rate, cyanosis, chest pain, cough, and hemoptysis but these may be absent in the anesthetized, mechanically ventilated patient. Instead, hypoxia, hypotension, swollen neck veins, pulmonary hypertension, increase in central venous pressure, and decrease in end-tidal CO2 can be used to diagnose PE. In severe cases, PE can cause cardiopulmonary collapse leading to cardiac arrest [2,4]. Therefore, one must suspect PE when a patient suddenly develops dyspnea, hypotension, hypoxia, and cyanosis proceeding to cardiac arrest. Patients with an acute episode of venous thromboembolism in the three to six months prior to surgery are usually given a long-term anticoagulant therapy. The recurrence of venous thromboembolism increases with cessation of anticoagulant therapy during the perioperative period. The recurrence rate of venous thromboembolism is about 40% in the first month and 10% during the second month but anticoagulation reduces the risk of recurrent venous thromboembolism by about 80% [5]. Therefore, elective surgery should be avoided in the first month after an acute episode of venous thromboembolism. If delaying surgery is not possible, intravenous heparin should be given before and after the surgery and a vena caval filter insertion is recommended for those patient with high risk of bleeding and history of thromboembolism within the previous month [5,6].
After sustaining hip and tibia fracture, this patient developed PE 8 days before surgery and was under heparin treatment. For spinal anesthesia and operation, heparin infusion was stopped a day before surgery at midnight and the development of deep vein thrombosis could have occurred during this period of heparin cessation. During the 40 min between spinal anesthesia and operation, vital signs were stable and the patient did not complain of any discomfort. The patient suddenly developed hypotension and loss of consciousness immediately after tourniquet inflation. Therefore, it is possible that the mechanical stress of tourniquet inflation dislodged preformed venous thrombi leading to this patient's PE. Elsewhere, it has been reported that the application of the Esmarch bandage or deflation of tourniquet caused venous thrombi to dislodge and subsequent PE [7,8]. But case reports also exist where a tourniquet inflation without prior use of an Esmarch bandage caused bradycardia, unresponsiveness, and cardiac arrest with pulmonary emboli found at autopsy [9-11].
Regional anesthesia is associated with a reduction in the incidence of venous thromboembolism and PE compared to general anesthesia [12,13]. But this only means that the incidence of new thrombi formation is reduced [14] and regional anesthesia might have a different influence if there is a recent history of thromboembolism. Lee et al. [14] reported a case of PE in a 55-yr-old male under spinal anesthesia for femur fracture where venous thrombus may have been dislodged from the vessel due to peripheral vasodilation and fluid loading before and during spinal anesthesia. In our case, the surgical circumstances were similar and it may be that the mechanical stress of tourniquet inflation contributed to the dislodging of the thrombus.
The American College of Chest Physicians (ACCP) guidelines recommend routine pharmacologic prophylaxis for patients undergoing orthopedic surgery with thromboembolic risk factors. Pharmacologic options include low-molecular-weight heparin (LMWH), fondaparinux (Factor Xa inhibitor), or vitamin K antagonist use started preoperatively and continued as early as possible following surgery. Especially for hip fracture patients in whom surgery is likely to be delayed, unfractionated heparin or LMWH are recommended as prophylaxis against thromboembolism during the time between hospital admission and surgery. Also recommended are mechanical compression devices like intermittent pneumatic compression stockings, graduated compression stockings and venous foot pumps [15].
This patient presented with old age and hip and tibia fracture. Despite the absence of venous thrombus on preoperative venography, he was a high-risk orthopedic patient as he had a history of recent PE treated with heparin and his operation was scheduled for 11 days after the accident. It may have been an error not to place an inferior vena caval filter for this patient. Therefore, high-risk patients for PE need active prophylaxis against thromboembolism and thorough monitoring during manipulation of the lower legs, especially those receiving operation within one month after PE.
In this patient, PE was diagnosed by TEE and cardiac arrest was successfully treated by cardiopulmonary resuscitation. During anesthesia, patient's positional change, leg elevation, tourniquet inflation/deflation and limb exsanguination by an Esmarch bandage need intensive monitoring especially in patients with trauma of the lower extremities and history of PE. Preoperative anticoagulation and diagnostic workup is necessary to exclude the possibility of venous thrombosis but it does not prevent thromboembolism in 100% of patients. Therefore, a high index of suspicion for the presence of deep vein thrombus must be maintained throughout the perioperative period.
References
1. Murray MJ, Coursin DB, Pearl RG, Prough DS. Critical care medicine: perioperative management. 1997, Philadelphia, Lippincott-Raven Publishers. pp 575-587.
2. Dehring DJ, Arens JF. Pulmonary thromboembolism: disease recognition and patient management. Anesthesiology 1990; 73: 146-164. PMID: 2193557.


3. Jang IS, Kim HT, An SK, Kwon YE, Lee JH. Pulmonary thromboembolism occurred immediately after leg elevation under induction of general anesthesia in a patient with femur fracture. Anesth Pain Med 2009; 4: 129-132.
4. Barnes PJ, Liu SF. Regulation of pulmonary vascular tone. Pharmacol Rev 1995; 47: 87-131. PMID: 7784481.

5. Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336: 1506-1511. PMID: 9154771.


7. Parmet JL, Berman AT, Horrow JC, Harding S, Rosenberg H. Thromboembolism coincident with tourniquet deflation during total knee arthroplasty. Lancet 1993; 341: 1057-1058. PMID: 8096961.


8. Cohen JD, Keslin JS, Nili M, Yosipovitch Z, Gassner S. Massive pulmonary embolism and tourniquet deflation. Anesth Analg 1994; 79: 583-585. PMID: 8067569.


9. San Juan AC Jr, Stanley TH. Pulmonary embolism after tourniquet inflation. Anesth Analg 1984; 63: 374PMID: 6703361.

10. Hofmann AA, Wyatt RW. Fatal pulmonary embolism following tourniquet inflation. J Bone Joint Surg Am 1985; 67: 633-634. PMID: 3980509.


11. Araki S, Uchiyama M. Fatal pulmonary embolism following tourniquet inflation. Acta Orthop Scand 1991; 62: 488PMID: 1950496.


12. Bullingham A, Strunin L. Prevention of postoperative venous thromboembolism. Br J Anaesth 1995; 75: 622-630. PMID: 7577292.



13. Haas SB. Effects of epidural anesthesia on incidence of venous thromboembolism following joint replacement. Orthopedics 1994; 17(Suppl): 18-20. PMID: 7937384.


14. Lee JJ, Shin BS, Hong JS. Pulmonary thromboembolism following spinal anesthesia. Korean J Anesthesiol 1999; 36: 534-539.

15. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133: 381S-453S. PMID: 18574271.


- TOOLS