The interaction of intrathecal neostigmine and N6-cyclohexyladenosine on anti-allodynic effects in rats with a nerve ligation injury
Article information
Abstract
Background
Nerve ligation injury in rats produces a pain syndrome that includes mechanical allodynia. Intrathecal administration of cholinesterase inhibitors or adenosine receptor agonists have anti-allodynic effects in this model. Therefore, we tested the interaction between intrathecal neostigmine and N6-cyclohexyladenosine (CHA) in a rat behavioral model of neuropathic pain.
Methods
Male Sprague-Dawley rats were prepared with tight ligation of the spinal nerves for producing allodynia and with a lumbar intrathecal catheter for drug administration. Allodynia thresholds for hindpaw withdrawal against mechanical stimuli were assessed and converted to percent maximal possible effect. Neostigmine (0.3-10 µg) and CHA (0.03-3 µg) were administered to obtain the dose-response curves and the 50% effective dose (ED50). Equal fractions (1/2, 1/4 and 1/8 ED50s) of the two drugs were administered to establish the ED50 of neostigmine-CHA combination. Side effects were also assessed. The drug interaction was evaluated by isobolographic and fractional analyses.
Results
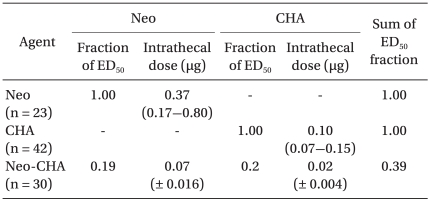
Neostigmine, CHA, and the neostigmine-CHA combination dose-dependently produced anti-allodynia effects. Side effects such as sedation and motor weakness were similar in the three groups. In the isobolographic analysis, the experimental ED50 for the combination of neostigmine-CHA lay far below and to the left of the theoretical additive line. Fractional analysis indicated that the total combination fraction of the two drugs was 0.39.
Conclusions
Intrathecal co-administration of neostigmine and CHA showed a synergistic anti-allodynia effect.
Introduction
Peripheral nerve injury may produce a pain syndrome consisting of hyperalgesia, spontaneous pain, and mechanical and thermal allodynia. Unilateral tight ligation of the fifth and sixth lumbar (L5 and L6) spinal nerves in rats produces signs that appear representative of neuropathic pain [1]. The spinal nerve ligation (SNL) model displayed profound and long-lasting mechanical allodynia that can be reduced by sympathectomy [2]. Cholinesterase inhibitors and adenosine receptor agonists produce an anti-allodynic effects in rats with a nerve ligation injury [3,4]. Intrathecal (IT) administration of neostigmine, a cholinesterase inhibitor, has an anti-nociceptive effect [3]. IT injection of N6-cyclohexyladenosine (CHA), an adenosine A1 receptor agonist, also produces anti-nociception in the neuropathic rat model [5]. Although the synergistic interaction of spinal neostigmine and adenosine has been demonstrated in a rat model of postoperative hypersensitivity [6], there have been no studies on the anti-allodynic interaction between spinal neostigmine and CHA in the SNL model. Therefore, we assessed the drug interaction using isobolographic and fractional analyses between IT neostigmine and CHA in the SNL model.
Materials and Methods
Animal preparation
The experiments were performed under a protocol approved by the Animal Care Committee. Male Sprague-Dawley rats (weight 160-180 g) were housed individually in a temperature-controlled (21 ± 1℃) vivarium and allowed to acclimate for three days in a 12/12-h day/night cycle.
Surgical procedures
To create the SNL model, a surgical procedure was performed according to the method described by Kim and Chung [1]. Under the enflurane anesthesia, the left L5 and L6 spinal nerves were isolated and ligated tightly with 6-0 black silk distal to the dorsal root ganglion and proximal to the formation of the sciatic nerve. The IT catheter was implanted if the rat showed a withdrawal threshold of 4.0 g or less by postoperative day 7, which indicated mechanical allodynia. The implantation of IT catheter was performed as previously described [7]. Under enflurane anesthesia, a polyethylene tube (PE-10) was passed 8.5 cm caudally from the cisterna magna to the rostral edge of the lumbar enlargement and externalized through the skin. Only animals without the evidence of neurologic deficit after the operation were included in this study. Rats were kept in individual vivaria and allowed to recover for 5 days after catheter implantation.
Behavioral measures
Behavioral testing was done at the same time during the day (9:00 AM to 4:00 PM). Rats were placed in individual plastic cages with wire mesh bottoms and allowed to acclimate for 20 min. The mechanical threshold was measured by applying a von Frey filament (Stoelting, Wood Dale, IL, USA) vertically to the midplantar surface of the hindpaw ipsilateral to the nerve ligation injury until a positive sign for pain behavior was elicited. According to the method described by Chaplan et al. [8], a series of eight calibrated von Frey filaments (0.41, 0.70, 1.20, 2.00, 3.63, 5.50, 8.50, and 15.10 g) was applied serially to the paw in ascending order of strength with sufficient force to cause gentle bending against the paw and held for six seconds. A sharply withdrawal or paw flinching was considered a positive response. The mechanical stimulus producing a 50% likelihood of withdrawal was determined by using the up-down method. Measurements were taken before and 15, 30, 60, 90, and 120 min after the IT administration of each drug. Side effects were simply evaluated by observing the presence of sedation and motor weakness. Severe sedation was defined as a significant decrease in spontaneous activity and a loss of the orienting reflex to light touch. Motor weakness was assessed by observing abnormal ambulation, abnormal weight bearing, or righting and stepping reflexes.
Drugs
The following drugs were used in this study: neostigmine bromide (Research Biochemicals International [RBI], Natick, MA, USA), CHA (RBI), and pirenzepine (RBI). All drugs were dissolved in normal saline and were administered intrathecally with a microinjection syringe (Hamilton Co., Reno, NV, USA) over a 60 second interval in a volume of 10 µl, followed by a 10-µl flush.
Experimental paradigm
The first series of experiments defined the dose-response curves of intrathecal administered neostigmine (0.3, 1, 3, and 10 µg) and CHA (0.03, 0.1, 0.3, 1, and 3 µg) from the mean percent maximal possible effect (%MPE) to establish the ED50s of two drugs. In the second series of experiments, ED50 fractions (1/2, 1/4, and 1/8) of each drug were administered concurrently to establish the ED50 of neostigmine-CHA combinations. Thereafter, the drug interactions were evaluated by isobolographic and fractional analyses. In the third series of experiments, to identify a possible mechanism of the interaction between neostigmine and CHA, muscarinic antagonist pirenzepine 3 µg was administered intrathecally 10 min before the injection of both drugs.
Isobolographic and fractional analysis
To determine whether the drug interaction between neostigmine and CHA is additive or synergistic, an equal dose ratio isobolographic analysis was conducted by using the method of Tallarida and Murray [9]. The theoretical additive combination dose was calculated by the method described by Tallarida et al. [9,10]. The experimental ED50 values were compared with the theoretical additive ED50 values as defined by the theoretical additive line. The theoretical additive ED50 point lies on a line connecting the ED50 values of the individual drugs. The experimentally derived value that lies below and to the left of the theoretical additive line is considered to be synergistic, whereas value that lies above and to the right of the line demonstrates an antagonistic interaction.
Fractional analysis was performed to obtain a value for describing the magnitude of the drug interaction. A total fraction was calculated with the following formula: (ED50 dose of neostigmine in combination / ED50 value for neostigmine alone) + (ED50 dose of CHA in combination / ED50 value for CHA alone). A value near 1 implies an additive interaction, and value less than 1 indicates a synergistic interaction [11].
Data analysis and statistics
The peak drug effects were recorded and then used to calculate %MPE values. These %MPE values were plotted versus the log dose for dose response data. The withdrawal threshold data from von Frey filament testing were obtained as the actual threshold in grams and converted to %MPE values by the following formula: %MPE for anti-allodynia = ([post-drug threshold - baseline threshold] / [15 g - baseline threshold]) × 100. The cut-off value was defined as a stimulus intensity of 15 g for the mechanical threshold (i.e., %MPE = 100).
Data are presented as mean ± SEM or 95% confidence intervals (CIs). The ED50 values, slopes, and 95% CIs were determined using a dose-response analysis. Variances and their 95% CIs for the theoretical additive ED50 were calculated from the variances of each component administered alone. The difference between the theoretical additive point and the experimentally derived ED50 was compared by a Student t-test. The effect of drugs on mechanical allodynia was tested by one-way analysis of variance for repeated measures followed by Dunn's post hoc test. The difference between the agonistic effect and the antagonistic effect was tested by Mann-Whitney Rank Sum test. P value < 0.05 was considered statistically significant.
Results
Baseline response characteristics
After spinal nerve ligation, all rats displayed a significant decrease in mechanical threshold (from 15 g to the range of 1 to 4 g) necessary to evoke a brisk withdrawal response in the injured hindpaw in response to von Frey filament stimulation.
Anti-allodynic effects of drugs
IT administration of neostigmine, CHA, and their combination produced a dose-dependent anti-allodynic effect (Fig. 1). The maximal anti-allodynia effects occurred within 30 min, and then decreased gradually to baseline with the passage of time. The patterns of time-effect course were similar in all groups (data not shown).

Dose-response curves from the peak effects of percent maximal possible effect (%MPE) for anti-allodynia in the neostigmine, N6-cyclohexyladenosine, and their combination subgroups. These curves show a dose-dependent anti-allodynic effect. Data are expressed as mean ± SEM. Doses (µg) are represented logarithmically on the x axis and peak %MPE of each group is represented on the y axis. CHA: N6-cyclohexyladenosine, Neo: neostigmine, Neo-CHA: combination of neostigmine and N6-cyclohexyladenosine. *P < 0.05 compared with baseline value in each group.
Drug interaction
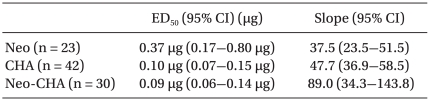
The ED50 values and slopes of neostigmine, CHA, and their combination are described in Table 1. The dose-response curve of the combination group was shifted to the left and steeper compared drug alone (Fig. 1). Isobolographic analysis indicated a synergistic interaction between neostigmine and CHA (Fig. 2). The experimentally determined mixture ED50 (± SEM) was 0.07 µg (±0.016) for neostigmine and 0.02 µg (±0.004) for CHA. The theoretical additive ED50 was calculated to be 0.18 µg (±0.04) for neostigmine and 0.05 µg (±0.01) for CHA. The experimental value of neostigmine-CHA combination was significantly less than the calculated additive value (P < 0.05). The total fraction for the neostigmine-CHA combination was 0.39, indicating a synergistic interaction (Table 2).

Isobologram for the interaction between intrathecal neostigmine and N6-cyclohexyladenosine. Horizontal and vertical bars indicate SEM. The diagonal line connecting two 50% effective dose (ED50) points is the theoretical additive line. The ED50 point A is calculated from the ED50 values and 95% confidence intervals of each drug. The experimental ED50 point B lies far below the line of additivity, indicating significant synergism. CHA: N6-cyclohexyladenosine, Neo: neostigmine. *P < 0.05 compared with theoretical ED50 point A.
Antagonistic study
Pretreatment with the muscarinic M1 antagonist pirenzepine significantly reduced the anti-allodynic effect of IT neostigmine-CHA combination (P < 0.05, Fig. 3).

Antagonistic study of the combination subgroup by pirenzepine. Pretreatment with the muscarinic M1 receptor antagonist, pirenzepine, decreases the anti-allodynic effect. Data are expressed as mean ± SEM. ED50 = 50% effective dose, Neo-CHA: combination of neostigmine and N6-cyclohexyladenosine, Pir: pirenzepine. *P < 0.05 compared with baseline value in each group. †P < 0.05 compared with pirenzepine pretreatment group. ‡P < 0.05 compared with control group.
Side effects
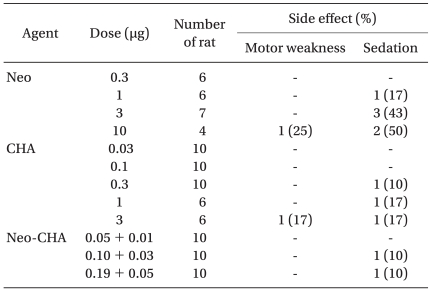
Some rats in all groups displayed a mild to moderate sedation and motor weakness, but no severe sedation or motor weakness. There was no apparent increase in the incidence and severity of side effects in the combination group (Table 3). After IT administration of either 10 µg of neostigmine or 3 µg of CHA, a moderate motor weakness was observed in two rats (one in the neostigmine group and one in the CHA group).
Discussion
After peripheral nerve injury, a harmless, low-intensity mechanical stimulus can elicit pain behavior mediated by the activation of low threshold mechanoreceptors subserved by large myelinated primary afferents [12]. The mechanisms underlying this miscoding of low-threshold afferent information could result from spontaneous activity in the dorsal root ganglion [13], trans-synaptic degenerative changes of the dorsal horn neurons [14], abnormal sympathetic innervation of the dorsal root ganglion neurons [15], and sprouting of numerous large myelinated sensory fibers into lamina II, an area where they do not normally terminate [16].
The spinal cholinergic muscarinic system is important for the regulation of nociception. Cholinesterase inhibitors or muscarinic receptor agonists produce an anti-allodynic effect that can be blocked by muscarinic antagonists [3]. Furthermore, autoradiography and immunohistochemical studies have shown a high density of spinal muscarinic receptors in the superficial laminae, areas which were involved predominantly with the processing of afferent pain impulses [17,18]. Muscarinic agonists may work by direct postsynaptic hyperpolarization of the dorsal horn neuron by opening G protein-coupled inwardly rectifying K+ channels [19] or activation of muscarinic receptors on GABAergic interneurons to stimulate GABA release, which then reduces glutamate release onto lamina II neurons [20]. Either way, the spinal cholinergic muscarinic system is intrinsic and regulates afferent input.
The spinal muscarinic system tonically inhibits noxious mechanical, but not noxious thermal, transmission [21]. Here, IT neostigmine dose-dependently produced an anti-allodynic effect and pretreatment with the muscarinic antagonist, pirenzepine, significantly diminished the anti-allodynic effect of the neostigmine-CHA combination. Therefore, spinal muscarinic stimulation may modulate the transmission of afferent allodynic information.
Adenosine also modulates nociceptive transmission in the spinal cord. Both A1 and A2 subtypes of adenosine receptors are present in the substantia gelatinosa of the spinal dorsal horn [22,23]. Although adenosine A2 agonists have no anti-nociceptive effect, adenosine A1 receptors produce anti-nociception in the spinal cord [5]. Adenosine A1 receptor agonists attenuated not only postoperative hyperalgesia but also mechanical allodynia [4,6]. Spinal adenosine receptor stimulation can have anti-allodynic activity by presynaptic inhibition of excitatory neurotransmitter release [24], postsynaptic inhibition of the effects of excitatory neurotransmitters [25], and spinal norepinephrine release leading to analgesia by α2-adrenoceptors [26]. These results suggest that adenosine receptor agonists can modulate the underlying spinal hyperexcitability state involved in neuropathic pain.
Chiari and Eisenach [6] reported that IT neostigmine interacted synergistically with adenosine to reduce postoperative hypersensitivity, reflecting an adrenergic component of adenosine mechanism of action in the postoperative model. Spinal adenosine A1 receptor activation induced dorsal horn norepinephrine release, ultimately leading to analgesia by an α2-adrenoceptor mechanism [27]. IT administration of an α2-adrenoceptor agonist, when combined with muscarinic receptor agonist, produces a synergistic anti-allodynic effect in the SNL model [28]. The anti-nociceptive action of adenosine A1 receptor agonists results from inhibition of excitatory neurons such as substance P or NMDA [29], whereas cholinesterase inhibitors regulate the GABA inhibitory system [20]. Therefore, our results may reflect both an adrenergic component of adenosine activity as well as other interactions between cholinesterase inhibitors and adenosine A1 receptor agonists.
The antiallodynic effects of intrathecally co-administered drugs may be mediated by independent receptor systems and there was a significant dose reduction of each drug. Synergism usually indicates that two drugs have different final pathways to produce their effects, but can also result from a decreased clearance, changes in agonist affinity, and functional interactions. The duration of activity was not changed in the combination group, suggesting there was no change in the clearance of either drug. Changes in agonist affinity can be reflected by changes in slopes of dose-response curves [30]. Here, the slope was increased and shifted to the left in the combination group (Fig. 1). Functional receptor interactions should increase the appearance of sedation and motor weakness as well as the allodynic component, but this was not observed, excluding a facilitation of receptor interactions (Table 3).
Pretreatment with pirenzepine, a muscarinic antagonist, significantly reduced the maximal effect of neostigmine-CHA combination. We chose pirenzepine because its antagonistic effect was most effective in the reversal of mechanical allodynia by IT neostigmine in the SNL model [3]. This finding suggests that the spinal cholinergic system is necessary for a synergistic anti-allodynic interaction between IT neostigmine and CHA.
Although not quantified systematically, IT neostigmine or CHA dose-dependently reduced spontaneous activity. Some rats showed a mild to moderate degree of side effects, but none of them showed severe sedation or motor weakness, and it was difficult to measure the degree of drug interaction on side effect profiles (Table 3).
In conclusion, intrathecally administered neostigmine or CHA produced a dose-dependent anti-allodynia without severe side effects. IT co-administration of neostigmine and CHA showed a synergistic anti-allodynic effect on allodynia after nerve ligation injury.