Introduction
Sevoflurane is an anesthetic used in pediatric patients, which allows fast and smooth anesthesia induction, as well as a fast recovery [
1-
3]. However, it is reported to also have a higher incidence rate of emergence agitation [
1-
5]. Pediatric patients who undergo strabismus surgery have their eyes covered after the surgery, so they experience greater agitation. Preoperative treatment for patients includes reducing in anxiety and helping make the process entering the OR and anesthesia induction easy [
6,
7]. Preoperative treatment also aims at reducing the emergence agitation occurring during recovery. Sedatives like benzodiazepine are used for preoperative treatment [
6-
9]. Arai et al. [
8] reported that they were able to reduce emergence agitation more in patients with the combination of midazolam and diazepam as preoperative treatment than with only midazolam. This is because diazepam has a longer action time than midazolam. Such research studies show that the difference of remaining midazolam concentration at the effective site during the recovery time plays an important role. This study aimed at seeing how administering midazolam during anesthesia recovery can accurately show the effects of midazolam in emergence agitation, as well as at finding the proper dose of midazolam with minimum disturbance to the patient's recovery rate.
Materials and Methods
Sixty pediatric patients of both genders who were presented for strabismus surgery and satisfied the inclusion criteria were enrolled for the present study. The inclusion criteria were that the patients must be at around the age from 2 to 7, and have ASA class 1. After the ethics committee of our institution approved of the study, written informed consent was obtained from the parents of the patient-subjects.
The patients were randomly placed in four groups from Group I to Group IV. Before the end of the surgery, we stopped administering sevoflurane and N
2O and injected normal saline 2 ml in Group I and Group IV. We administered a 2 ml mixture of midazolam 0.025 mg/kg, midazolam 0.050 mg/kg, and normal saline to Group II and Group III. Before the patients were moved to the operating room, one anesthetist recorded agitation scores (
Table 1) [
2] on a scale from 1 to 5. Preoperative treatment was not given to any group. In the operating room (OR), we started anesthesia induction by an intravenous injection of atropine 0.01 mg/kg, thiopental sodium 5 mg/kg, and vecuronium 0.1 mg/kg, and then we started intubation. We administered N
2O 1.5 L/min, O
2 1.5 L/min, and sevoflurane 2-3 vol%, and we kept the proper anesthetic depth in the surgery. To manage pain post-surgery, we stopped administering sevoflurane and N
2O, and at the same time, administered ketorolac 0.8 mg/kg to all patients. We then removed air and the contents of the stomach using a suction tube.
After the surgery, we stopped administering N2O and only gave 100% O2. As an antagonism of muscle relaxation, we used glycopyrolate and pyridostigmine. The patients were able to move their extremities. The ventilation volume was adequate. Every 30 seconds, they were given the verbal command 'open your eyes'. When the patients were able to open their eyes, we extubated the tracheal tube. We measured the time from when we stopped giving sevoflurane to the time of extubation.
We then immediately moved the patients to the postoperative care unit (PACU) and kept their guardians with them. Once they were in the PACU, the anesthetist recorded their agitation scores. We did not give the anesthetist information on which group the patient belonged to or informed him of the medication the patients received. Among the patients with agitation scores 4 or 5, Group IV patients were given by i.v. a 1 ml mixture of midazolam 0.025 mg/kg and normal saline. When the agitation score did not decrease after 3 minutes, we administered the same ingredient mixture by i.v. a total of 3 times. In the remaining 3 groups, we administered normal saline 1 ml by IV.
In the PACU, if the agitation scores changed, we recorded how long each agitation score lasted. The length of the period of the emergence agitation lasted (when it was 4 or 5) out of the length of stay in the PACU was recorded. We also recorded how long the post-anesthetic recovery score (PAR score) took to go up to 10, as well as the agitation scores just before the patients were transferred to the ward. When a patient's score reached 10, he was transferred to the ward.
We presented the data as the mean ┬▒ SD. We analyzed the categorical data using Chi-square followed by a Fisher's exact test. We analyzed the continuous data by one-way ANOVA followed by Tukey post hoc test. Statistical significance was defined as P < 0.05.
Results
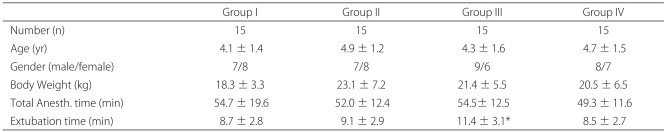
The data on the mean age, body weight, duration of the operation, and gender were similar for all three groups (
Table 2). After we stopped administering sevoflurane, we extubated the endotracheal tube, which took significantly longer in Group III than it did in Group I (
Table 2).
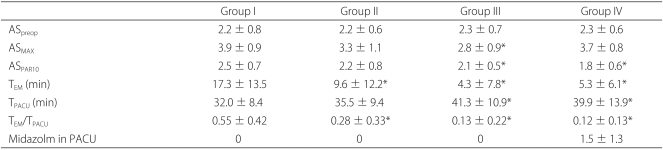
Before entering the OR, there was no significant difference in agitation scores among all the groups (
Table 3). After the surgery, we compared the highest agitation scores recorded in the PACU, which showed only Group III to have a significantly low score. Group III and Group IV had significantly low agitation scores before the patients' PAR scores were 10 and they were moved to the PACU, as well as significantly short lengths of stay in the PACU (
Table 3).
Group I, compared to the other groups, had significantly higher agitation scores while in the PACU. The rate of the period when the agitation score was 4 or 5 out of the period of stay in the PACU was 0.55 ┬▒ 0.42 in Group I, 0.28 ┬▒ 0.33 in Group II, 0.13 ┬▒ 0.22 in Group III, and 0.12 ┬▒ 0.13 in Group IV. Compared to Group I, the other groups had significantly low rates (
Table 3). While the patients were in the PACU, we administered midazolam in Group IV to 3 times to 4 patients, 2 times to 5 patients, and none to 6 patients. The average administration rate of midazolam was 1.5 ┬▒ 1.3 times (
Table 3).
Discussion
Pediatric patients in foreign, frightening environments are more likely to fall into a state of agitation and are less cooperative than adult patients. It is worse when the pediatric patients wake up in the PACU from general anesthesia. Thus the medical staff has a hard time taking care of pediatric patients in the PACU [
1,
3]. Recent developments in anesthetics have made it possible for rapid anesthesia induction and rapid recovery. However, the occurrence rate of emergence agitation has nevertheless increased [
10]. Patients waking up from general anesthesia usually experience a rather disturbed and excited emergence agitation, but the symptoms are worse for pediatric patients [
1-
3]. They cry heavily and writhe (to free themselves), pull on their IV line, and impose a heavy burden on the medical staff. They play a big role in making their parents lose confidence and satisfaction in the anesthetization and surgery [
3].
It is believed that many factors affect emergence agitation. Pain, foreign environments, anxiety/excitement before anesthesia induction, and the special characteristics of anesthetics are the main known factors [
3,
11-
13]. Emergence agitation is especially severe in patients who had strabismus surgery, because they have their eyes covered post-surgery. Bearing this in mind, we conducted the present study specifically on patients undergoing strabismus surgery. We administered ketrolac to all the patients after the surgery was over in order to eliminate all possible factors that are related to pain. It is known that pediatric patients who experience separation anxiety from their parents when entering the operating room and those who strongly resist during anesthesia induction have the highest incidence rate of emergence agitation [
12,
13]. The patients in our study had slightly higher emergence agitation rates, assumedly because we did not give them preoperative treatment. Sevoflurane is often the preferred choice for pediatric patients and it is mainly used for inhalation induction, because sevoflurane allows for smooth anesthesia induction and rapid recovery [
4,
5]. However, despite sevoflurane's strengths, it is associated with a higher rate of emergence agitation, which is even more prevalent in preschool boys [
1,
10,
14,
15]. Many research studies have been done on preventing emergence agitation include administering sedatives and analgesics, changing the types of anesthetics, and taking other different methods in reducing emergence agitation [
6,
8,
9,
11,
15,
16]. Lapin et al. [
17] reportedly reduced the emergence agitation rates in patients who underwent sevoflurane anesthesia with by administering midazolam pre-surgery.
In contrast, Breschan et al. [
16] reported that midazolam does not reduce the incidence rate of emergence agitation in sevoflurane anesthesia cases. Arai et al. [
8] stated that using midazolam together with diazepam was effective in reducing the rate of emergence agitation rather than using midazolam alone. Diazepam has a long half life and its onset time is slow, so its effects may be longer lasting compared to midazolam, which has a relatively shorter half-life and onset time [
1,
8]. So administering only midazolam when a surgery lasts for many hours may appear to have no effect on inhibiting emergence agitation.
Taking the above into consideration, we believed we could prove the effects of midazolam against emergence agitation by administering midazolam when the anesthesia wears off. In a pilot study, we administered various amounts of midazolam and checked responses before selecting the most appropriate administration method for this study. In Group II, we stopped administering sevoflurane at the time we gave midazolam 5 mg/kg to Group II patients. This did not affect the patients' anesthesia recovery rates at all. Although it did reduce emergence agitation, the level of reduction was not satisfactory. In Group III, we administered midazolam 0.05 mg/kg. Although their recovery was a bit slow, we found that midazolam effectively reduced emergence agitation.
We administered midazolam again on certain patients in the recovery room depending on their responses. They were given 0.25 mg/kg on the second time. They also similarly experienced anti-stimulation but also experienced the same, slightly slow recovery.
The emergence agitation rate while in the PACU (the length of period when the agitation scores were 4 or 5/the length of stay in the PACU) for the control group, Group I, was 0.55 ┬▒ 0.42, indicating that the emergence agitation signs were apparent for more than half of the period of stay in the PACU. But Group II's rate was 0.28 ┬▒ 0.33, Group III's rate was 0.13 ┬▒ 0.22, and Group IV's rate was significantly low at 0.12 ┬▒ 0.13. The length of stay in the PACU was for the control group, Group I, 32.0 ┬▒ 8.4 minutes, whereas in Group II, it was 35.5 ┬▒ 9.4 minutes, in Group III it was 41.3 ┬▒ 10.9 minutes, and in Group IV, it was 39.9 ┬▒ 13.9 minutes. At maximum, the length of stay was only extended for about 10 minutes. Moreover, there were no side effects such as respiratory depression, airway obstruction, and bradycardia, whatsoever.
The results of the study clearly indicate that midazolam has the effect of reducing emergence agitation. Although more study is needed on administration methods and appropriate timing for administration, the results so far show that we should administer the usual dose of midazolam as preoperative treatment. If a surgery lasts for a long time or no midazolam is administered before anesthetization, we should administer midazolam 0.025 mg/kg the moment when the anesthetics are discontinued. When the patient has emergence agitation while in the PACU, we consider the most effective method to be administering midazolam 0.025 mg/kg several times depending on the patient's response.
In conclusion, for pediatric patients under sevoflurane anesthesia, midazolam administration of 0.025 mg/kg or 0.05 mg/kg (at the moment the administration of sevoflurane is stopped at the end time of the surgery) and midazolam administration 0.025 mg/kg up to 3 times in the recovery room (depending on the responses of the pediatric patient) slightly prolonged the length of stay in the PACU. But it effectively reduced emergence agitation without any side effects.
Acknowledgements
This work was supported by the research grant of the Chungbuk National University in 2009.
References
1. Cote CJ. Edited by Miller RDPediatric anesthesia. Miller's anesthesia. 2009, 7th ed. : Philadelphia, Churchill Livingstone. pp 2565-2569.

2. An TH. The comparison of incidence of postoperative agitation in children after enflurane or sevoflurane inhalation anesthesia. Korean J Anesthesiol 2002; 43: 174-178.

3. Everett LL. Edited by Longnecker DE, Brown DL, Newman MF, Zapol WMAnesthesia for children. Anesthesiology. 2008, : New York, McGraw-Hill. p 1533.
4. Morgan GE, Mikhail MS, Murray MJ. Clinical anesthesiology. 2006, 4th ed. USA, McGraw-Hill. pp 173-174.
5. Johannesson GP, Flor├®n M, Lindahl SG. Sevoflurane for ENT-surgery in children. A comparision with halothane. Acta Anaesthesiol Scand 1995; 39: 546-550. PMID:
7676795.

6. Kararmaz A, Kaya S, Turhanoglu S, Ozyilmaz MA. Oral ketamine premedication can prevent emergence agitation in children after desflurane anaesthesia. Paediatr Anaesth 2004; 14: 477-482. PMID:
15153210.

7. Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth 2006; 16: 748-753. PMID:
16879517.

8. Arai YC, Fukunaga K, Hirota S. Comparison of a combination of midazolam and diazepam and midazolam alone as oral premedication on preanesthetic and emergence condition in children. Acta Anaesthesiol Scand 2005; 49: 698-701. PMID:
15836687.

9. Tazeroualti N, De Groote F, De Hert S, De Vill├® A, Dierick A, Van der Linden P. Oral clonidine vs. midazolam in the prevention of sevoflurane-induced agitation in children. A prospective, randomized, controlled trial. Br J Anaesth 2007; 98: 667-671. PMID:
17416907.


10. Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery: a comparison with halothane. Paediatr Anaesth 2000; 10: 419-424. PMID:
10886700.

11. Aouad MT, Kanazi GE, Siddik-Sayyid SM, Gerges FJ, Rizk LB, Baraka AS. Preoperative caudal block prevents emergence agitation in children following sevoflurane anesthesia. Acta Anaesthesiol Scand 2005; 49: 300-304. PMID:
15752392.

12. Aono J, Mamiya K, Manabe M. Preoperative anxiety is associated with a high incidence of problematic behavior on emergence after halothane anesthesia in boys. Acta Anaesthesiol Scand 1999; 43: 542-544. PMID:
10342002.

13. Kain ZN, Caldwell-Andrews AA, Maranets I, McClain B, Gaal D, Mayes LC, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg 2004; 99: 1648-1654. PMID:
15562048.

14. Aono J, Ueda W, Mamiya K, Takimoto E, Manabe M. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997; 87: 1298-1300. PMID:
9416712.

15. Welborn LG, Hannallah RS, Norden JM, Ruttimann UE, Callan CM. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg 1996; 83: 917-920. PMID:
8895263.

16. Breschan C, Platzer M, Jost R, Stettner H, Likar R. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth 2007; 17: 347-352. PMID:
17359403.


17. Lapin SL, Auden SM, Goldsmith LJ, Reynolds AM. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatr Anaesth 1999; 9: 299-304. PMID:
10411764.